Abstract
The aim of this work was to investigate the optimal process conditions leading to high glucose yield (over 80 %) after wet explosion (WEx) pretreatment and enzymatic hydrolysis. The study focused on determining the “sweet spot” where the glucose yield obtained is optimized compared to the cost of the enzymes. WEx pretreatment was conducted at different temperatures, times, and oxygen concentrations to determine the best WEx pretreatment conditions for the most efficient enzymatic hydrolysis. Enzymatic hydrolysis was further optimized at the optimal conditions using central composite design of response surface methodology with respect to two variables: Cellic® CTec2 loading [5 to 40 mg enzyme protein (EP)/g glucan] and substrate concentration (SC) (5 to 20 %) at 50 °C for 72 h. The most efficient and economic conditions for corn stover conversion to glucose were obtained when wet-exploded at 170 °C for 20 min with 5.5 bar oxygen followed by enzymatic hydrolysis at 20 % SC and 15 mg EP/g glucan (5 filter paper units) resulting in a glucose yield of 84 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corn stover is abundantly available in the Midwest of USA and can be an excellent feedstock for biofuel production because of its lower lignin content compared to woody biomass. The commercial viability of biorefineries based on corn stover has been burdened by the use of expensive enzymes needed to hydrolyze the biomass material after pretreatment [1, 2]. It has been well established that producing higher concentration of sugars is an absolute necessity in an industrial setting as it lowers the heating requirements (lowering operating cost) and increases the volumetric efficiency (lowering capital cost) of the equipment [3]. Therefore, lowering the enzyme input and increasing the dry matter content during enzyme hydrolysis for higher cellulose conversion would be one of the most significant steps towards the direction of bioethanol production cost reduction and eventually leading to the commercialization of second-generation biorefineries based on the lignocellulosic feedstock.
Several researchers have worked on using corn stover for bioethanol production. Kaar et al. [4] used lime pretreatment followed by enzymatic hydrolysis at 5 % substrate concentration (SC) and 20 filter paper units (FPU) (Spezyme CP and Novozym 188) and obtained 60 % cellulose conversion. Kim et al. [5, 6] introduced ammonia recycle percolation pretreatment followed by enzyme hydrolysis at 1 % SC and 10 FPU [Spezyme CP and β-glucosidase (Sigma)] and obtained 92 % cellulose conversion. This concentration of solids will, however, be far from an industrial process. Bura et al. [7] used SO2-catalyzed pretreatment followed by enzyme hydrolysis at 8 % SC and 10 FPU (Spezyme CP, Novozym 188, and Multifect® Xylanase) and obtained 100 % cellulose conversion. However, again, the solid concentration was far lower than that needed for operating any industrial process. Using chemicals such as sulfur could further affect the downstream processing of products; for instance, sulfur will be attached to the solid fraction that remained after sugar extraction [8]. Recently, Yang et al. [9] used steam explosion pretreatment followed by enzyme hydrolysis at 25 % SC and 20 FPU (Celluclast) and obtained 85 % cellulose conversion. Even though this study achieved high glucose concentrations, the amount of enzymes used was higher, affecting the applicability of the process.
In this work, we will investigate the most efficient and cost-effective process conditions for producing sugars from corn stover using wet explosion (WEx) pretreatment, which only require oxygen and heat to open the structure of the biomass and which can operate at high SC [10, 11]. Our focus will be on finding the optimal conditions producing high yields of glucose while keeping the enzyme dose low—the “sweet spot.” As a first step, screening of WEx pretreatment will be performed to find the best pretreatment condition, which will be used for the optimization of enzymatic hydrolysis. The second step will be central composite design (CCD) to generate response surface, model fitting, and prediction of optimum parameters for enzymatic hydrolysis of WEx corn stover. The third step will be a scale-up study of enzymatic hydrolysis at optimized condition from lab scale to 100-L pilot scale. In addition, we will evaluate the process economics for determining the minimum enzyme dose, which still can support a biorefinery with corn stover as raw material.
Materials and Methods
Preparation of Biomass
Corn stover with a particle size of approximately quarter inch was kindly provided by Iowa State University, Ames, IA, USA. Before pretreatment, corn stover was milled to 2-mm particle size with a Retsch cutting mill SM 200 (Retsch Inc., PA, USA) and kept at room temperature. A portion of corn stover was milled to 1-mm particle size for the compositional analysis. The compositional analysis was performed in duplicates. The moisture content of the raw corn stover was found to be 8 %.
Experimental Approach
Figure 1 shows the experimental setup used in the present study. The milled corn stover was pretreated using WEx at 12 different pretreatment conditions with varying temperatures (160 to 180 °C), residence time (15 to 25 min), and oxygen pressure (2 to 6 bar). In order to investigate the efficiency of pretreatment, enzymatic digestibility test was conducted with Cellic® CTec2 (40 mg enzyme protein (EP)/g glucan) on all 12 pretreatment slurries. Sugars, sugar degradation products, and other fermentation inhibitors [hydroxymethylfurfurals (HMFs), furfural, and acetate] released during pretreatment or after enzymatic hydrolysis were analyzed using high-performance liquid chromatography (HPLC). Based on the sugar yield and the concentration of inhibitors, the optimal pretreatment condition was determined and used for finding the minimal enzyme dose needed for obtaining at least 80 % of the potential cellulose conversion from the biomass. This selected pretreatment was referred as the platform WEx pretreatment.
The enzymatic hydrolysis study to optimize the enzyme dosage was performed in shake flasks on the platform WEx-pretreated sample using varying SCs (5 to 20 %) and enzyme loading (5 to 40 mg EP/g glucan). Test of two parameters resulted in 14 experimental runs. The amount of free sugars released after these 14 enzymatic hydrolysis runs was analyzed using HPLC. Response surface methodology (RSM) was adopted to determine the optimal hydrolysis condition. Finally, the results for the optimal enzyme dose study were checked in a 100-L pilot-scale test at the optimal conditions (SC and enzyme loading) and the effect on the ultrastructure was followed using scanning electron microscopy to evaluate the changes done to the biomass during enzymatic hydrolysis. Finally, we analyzed the process economics to substantiate the effect of optimization study on the overall cost reduction of ethanol.
Measurement of Protein Assay and Cellulase Activity
The protein concentration of Cellic® CTec2 (kindly provided by Novozymes, Franklinton, NC, USA) was determined using standard Pierce® BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). The protein concentration in Cellic® CTec2 was measured as 210 mg/mL of enzyme. Filter paper activity of Cellic® CTec2 was measured according to enzyme activity assay [12]. The cellulolytic activity of concentrate was determined as 74 FPU/mL.
Selection of Platform WEx Pretreatment Parameters
WEx Pretreatment of Corn Stover
Corn stover was pretreated by WEx in a pilot plant at Washington State University as previously described [13]. The 12 different conditions used for the WEx pretreatment are shown in Table 1. Corn stover samples resulting from these pretreatment conditions are designated as CS-1 through CS-12. The 10-L WEx reactor was loaded with raw corn stover and water in proportions resulting in a total slurry of 5 kg with 25 % SC. After closing the reactor, oxygen was introduced into the reactor at pressures ranging from 2 to 6 bar. After introducing the oxygen, the pretreatment reactor was heated to temperatures varying from 160 to 180 °C with residence times at the desired temperature between 15 and 25 min (heating time, about 5 min). At the end of pretreatment reaction time, the pretreated biomass was flashed into a 100-L flash tank. The slurry was used for the optimization of enzymatic hydrolysis experiment (“Hydrolysis Testing”), and a small portion of the slurry was kept for solid and liquid analysis.
Hydrolysis Testing
The pretreated slurries from 12 different runs were hydrolyzed using Cellic® CTec2 with 40 mg EP/g glucan to investigate the maximum digestibility. A total of 50 g of well-mixed pretreated slurry with 25 % SC was taken for enzymatic hydrolysis. The enzymatic hydrolysis of the pretreated samples was performed at pH 5 and 50 °C for 72 h in 125-mL shake flasks. In order to overcome the mixing challenges of 25 % SC in the Erlenmeyer shake flasks, the pretreated samples were diluted to 20 % SC using 0.1 M sodium citrate buffer. Maximum care was taken to ensure that the solid/liquid ratio was maintained during sampling using intense mixing. The pretreated sample with the highest sugar concentration and the minimal concentration of sugar degradation products was selected for the enzymatic hydrolysis optimization studies. Experiment for the enzyme optimization was then designed as described in “Optimization of Enzymatic Hydrolysis.”
The glucose yield is determined by Eq. (1) as described below:
where (GluEH,L) is the glucose concentration (grams per liter) in enzymatic hydrolyzate liquid, \( {F}_{{\mathrm{cellulose}}_{\mathrm{RB}}} \) is the fraction of cellulose in the raw biomass as determined by compositional analysis, and (ini.sol) is the initial solid concentration (grams per liter) at which the enzymatic hydrolysis was performed.
Optimization of Enzymatic Hydrolysis
A 22 central composite design was used to optimize the enzymatic hydrolysis conditions. The statistical software Minitab (version 6.0, Minitab Inc., PA, USA) was used to generate the design of experiment, perform the statistical analysis, and develop the regression model. An enzyme loading of 5 to 40 mg EP/g glucan and SC 5 to 20 % were chosen as the independent variables. Fourteen experimental runs with six center points were conducted to evaluate the effect of enzyme loading and SC on glucose yield after enzymatic hydrolysis. Table 2 shows the design matrix and glucose yield response of the experiment. Data obtained from experimental runs were then analyzed according to the response surface regression and then fit to a second-order polynomial equation (Eq. (2)).
where Y i is the response value, β 0 is the constant coefficient, β i is the linear coefficients, β ij (i and j) is the interaction coefficients, β ii is the quadratic coefficients, and χ i is the variables. Analysis of variance (ANOVA) was used to estimate the significance of single factors, factors' square, and interaction of factors. The relationship between dependent and independent variables was further determined by the response surface plot.
Analytical Methods
A portion of the slurries obtained after WEx pretreatment and enzymatic hydrolysis were separated into solid and liquid fractions using a bench-top centrifuge (Eppendorf, Model 5804, 8,000 rpm, 10 min). The liquid portion was then decanted and filtered through a 0.45 μm syringe filter, and solids were washed and dried at 30 °C for 48 h. Free sugars in the liquid portion were analyzed using a Bio-Rad (Hercules, CA, USA) Aminex HPX-87P column with RI detector, operating at 83 °C with Milli-Q water (Barnstead Nanopure, USA) as an eluent with a flow rate of 1.0 mL/min. The composition of the solid fraction was determined as per established NREL method [14].
Scanning Electron Microscopy
The morphological features of raw, pretreated, and enzymatically treated corn stover were observed by scanning electron microscopy (SEM) (S-570, Hitachi Ltd., Japan) at an acceleration voltage of 10 kV. SEM was conducted under high vacuum to improve the conductivity of the samples and the resolution of the SEM micrographs.
Results and Discussions
Raw Material
The chemical composition of corn stover as determined using standard NREL protocols (“Analytical Methods”) showed the following compositions: glucan 38.67 % (±0.12), xylan 25.21 % (±0.03), galactan 1.83 % (±0.03), arabinan 2.85 % (±0.05), mannan 0.38 % (±0.16), Klason lignin 17.52 % (±0.14), acid-soluble lignin 1.50 % (±0.01), ash 2.57 % (±0.08), and acetate 4.34 % (±0.01) on dry basis. The composition analysis was performed in duplicates, and numbers shown are the average and the standard error is shown within the parentheses. The composition of raw material was used to calculate the glucose yields (percent) in the experiments.
Selection of Platform WEx Pretreatment Conditions
The amount of sugars released in the liquid phase after WEx pretreatment at 12 different conditions is shown in Fig. 2a. The amount of free sugars (glucose, xylose, and arabinose) released after WEx varied between 11.34 and 40.62 g/L. During the hydrolysis testing, the amount of free sugars released varied between 77.16 and 130.94 g/L (Fig. 2b). The amount of sugar degradation products (HMF and furfural) along with acetate (released from hemicellulose) generated during WEx varied between 3.79 and 12.79 g/L, whereas, during the enzymatic hydrolysis, their concentration varied between 6.50 and 15.49 g/L. The increase in HMF and furfural concentration during enzymatic hydrolysis is attributed to continued sugar degradation at a slower rate during enzyme hydrolysis. Interestingly, the pretreated sample CS-8 (170 °C, 20 min, 5.5 bar oxygen) released lower sugars during pretreatment (16.44 g/L) as compared to other pretreated samples which released maximum sugars during enzyme hydrolysis (130.94 g/L). These results are probably due to the fact that more oligomers were found in this sample (CS-8) due to a better opening of the structure (loosening of linkages between macromolecules in the cell wall). This is in agreement with previous studies by Ladisch et al. where they found that the optimal pretreatment conditions correlate with the oligosaccharides and not the monosaccharides released during pretreatment [15]. Sample CS-8 after pretreatment showed monomeric glucose (2.97 g/L), xylose (11.16 g/L), arabinose (2.31 g/L) and, after enzymatic hydrolysis, showed highest concentration of glucose monomers (102.61 g/L), xylose monomers (26.25 g/L), and arabinose (2.08 g/L), which gives amonomeric glucose yield of 81 % compared to the glucan concentration of the corn stover. The amount of degradation products in sample CS-8 after pretreatment was 3.99 g/L for HMF, 0.50 g/L for furfural, and 7.57 g/L for acetate. The amount of degradation products after enzymatic hydrolysis was 3.4 g/L for HMF, 0.46 g/L for furfural, and 9.6 g/L for acetate. The amount of lignin solubilized during pretreatment was 8 %, which remained unchanged during enzymatic hydrolysis. Sample CS-8 was, therefore, chosen as platform WEx pretreatment conditions for enzyme optimization study as it released highest sugars and minimal sugar degradation products.
Enzyme Optimization
Optimization of Enzymatic Hydrolysis Parameters
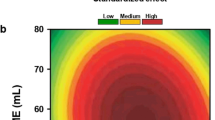
Full-factorial statistical model was developed by the statistical software Minitab 16. Table 2 shows coded and uncoded variables along with the experimental and model-predicted response. Low, midpoint, and high levels were coded as −1, 0 and +1, respectively. The response of glucose yield after 72 h of enzymatic hydrolysis from all 14 runs as a function of cellulase loading (mg EP/g glucan) and SC was evaluated in CCD. The interaction between response and variables was evaluated by response surface after 12, 24, 48, and 72 h (Fig. 3). Each response surface plot confirmed the interactions existing between the variables and glucose yield. As shown in Table 2, the experimental glucose yield matched closely with the model-predicted glucose yield with a regression coefficient of 97.68 %.
Minitab 16 was used to conduct ANOVA to evaluate individual and interactive effects of variables. Significance of coefficients of the model was analyzed. The results of the second-order response surface models for the glucose released during hydrolysis are presented in the form of ANOVA in Table 3. Equation (3) describes the second-order polynomial model to evaluate the relationship between variables and glucose yield of enzymatic hydrolysis.
In the above equation (Eq. (3)), Y is the glucose yield after 72 h of hydrolysis, χ 1 is SC (percent), and χ 2 is enzyme loading (milligrams EP per gram glucan). The statistical significance of the model was evaluated by F test for ANOVA, which confirmed that the regression was statistically significant. Temperature and pH were excluded from the optimization model because these factors had already been optimized as 50 °C and 5, respectively (www.bioenergy.novozymes.com).
Statistical effect of SC and enzyme loading on glucose yield is shown in Table 3. As shown in Table 3, the coefficient of regression (R 2) was calculated as 97.68 %, which indicated that the model is suitable to adequately represent the real relationships among the selected reaction variables. Highly adjusted R 2 (95.69 %) also showed the suitability of the model. Linear and squared terms of enzyme loading exerted a positive effect on the yield of glucose during enzymatic hydrolysis as the p values were <0.05. Linear SC has been found to have a significant effect; however, there was no direct effect of squared SC. This can be explained by the heterogeneity of the pretreated sample. Interaction of both enzyme loading and SC has a significant effect (p < 0.05), which indicates cumulative effect of both factors on enzymatic hydrolysis.
The objective of the optimization was to achieve a glucose yield of at least 80 %. The SC and enzyme loading combination at which the highest desirability (0.959) was obtained was chosen as the optimal conditions for the enzymatic hydrolysis with the predicted value of the response, 85.94 %. The optimal conditions for the enzymatic hydrolysis from the statistical analysis were found to be 20 % SC and 15 mg EP/g glucan (about 5 FPU), which gave a glucose yield of 84 % that is in agreement with the predicted response. Thus, we concluded that the model was valid and reliable in predicting the experimental results.
Influence of SC and Enzyme Loading During Enzymatic Hydrolysis
The significance of the quadratic coefficients of enzyme loading and SC for corn stover indicates a positive correlation between enzyme loading, SC, and hydrolysis yield. No noticeable mass transfer limitation was observed (due to high viscosity of the slurry) in contrast to previous studies [16, 17] as evident by high glucose yield. Optimal process conditions for enzymatic hydrolysis to high glucose included both high SC and low enzyme loading. This is a remarkable improvement over previous studies done with pretreated corn stover either at low SC (<10 %) or high enzyme loading (>10 FPU, equivalent to 30 mg EP/g glucan) to achieve higher cellulose conversion [5, 6, 18, 19]. By increasing the SC, the glucose yield improved significantly. This lowers the cost of fermentation and eliminates the need for concentration of the hydrolysate before fermentation, which would add to the overall cost.
Test at Scale-Up Conditions of Enzymatic Hydrolysis
Under the optimal conditions (20 % SC, 15 mg EP/g glucan, pH 5, and 50 °C), the model predicted a glucose yield of 84.8 %. The actual experiment yield was 84 %, which was in close agreement with the model's prediction, confirming the validity of the RSM model. A comparative study in 100-L pilot scale was performed with the optimized hydrolysis condition (20 % SC, 15 mg EP/g glucan) and compared to the hydrolysis condition at which the maximum yield was obtained (20 % SC, 40 mg EP/g glucan). Glucose yield of 84 and 99 % was attained at 15 and 40 mg EP/g glucan, respectively.
Scanning Electron Microscopy
SEM was performed to understand the morphological changes for the corn stover samples when hydrolyzed at the determined enzyme loading (15 mg EP/g glucan) versus the highest enzyme loading (40 mg EP/g glucan). Figure 4 shows SEM micrographs to elucidate the changes in the fibrillar structure of raw corn stover and after treatment (WEx and enzymatic hydrolysis). Corn stover (Fig. 4a) exhibited compact and highly ordered fibril structure covered with a wax layer, binding materials, and other deposits (common to herbaceous lignocellulosic biomass). Figure 4b showed damages in the fibrillar structure as a result of WEx pretreatment. Degradation caused by the harsh pretreatment conditions was demonstrated by the appearance of exposed smooth cellulose surface, peeling, reticular areas, and interfibrillar splitting in the direction of the fiber axis. Despite these major changes after WEx, some conducting vessels and pits still remained intact, demonstrating the need for enzyme digestion. Panels c and d of Fig. 4 show the SEM images after enzymatic hydrolysis conducted at enzyme loading of 15 and 40 mg EP/g glucan, respectively. Enzymatic hydrolysis caused the fibrous surface to be altered and enhanced the porosity induced by the WEx pretreatment. In both panels c and d of Fig. 4, degradation of fiber surface was significant, but the extent of degradation varied with the amount of enzyme protein added (and cellulase accessibility to fibers). With the determined optimal enzymes loading of 15 mg EP/g glucan (Fig. 4c), longitudinal disintegration of fibers and formation of fissures on the surface were observed and surface erosion was unevenly distributed, whereas with the increase in enzyme loading (Fig. 4d), uniform surface corrosion was observed. These observations lead to the conclusion that morphological differences between the corn stover samples hydrolyzed with 15 and 40 mg EP/g glucan were noticeable but relatively minor compared to the fact that 2.7 times more enzyme was added in the last case (Fig. 5).
Process Economics
Figure 6 shows the effect of varying enzyme loadings and SC on glucose yields. With the increase in the enzyme loading and SC, high glucose yield was achieved but, at the same time, enzyme cost increased significantly due to the need for higher enzyme dosage. Due to unavailability of an exact price of Cellic® CTec2, we found it more appropriate to use the relative cost savings derived from the quantity of enzymes used at the sweet spot for the economics modeling. Amount of enzymes (kilograms) was calculated from enzyme loading (milligrams enzyme protein per gram glucan) at respective substrate concentration for processing 1,000 kg of biomass slurry as basis as shown below.
Using 210 mg EP/mL of enzyme solution, enzyme density as 1.2 g/mL, SC 20 %, glucan 38.67 %, and enzyme loading at points A and B as 15 and 40 mg EP/g glucan, respectively, the amount of enzyme calculated at points A and B was 6.51 and 17.37 kg, respectively, to process 1,000 kg of biomass slurry. Glucose yields at points A and B were 84 and 99 %, respectively. At point A, our sweet spot, we observed a plateau, which suggests that further increase in enzyme loading and SC has little effect on the glucose yield, and therefore, we considered that point as optimal.
Comparing the relative difference in glucose yield and enzyme amount at points A and B, we found at the sweet spot (point A) a glucose yield that was 16 % lower; however, 62.5 % less enzymes were used, which would represent a significant saving compared to the optimal condition. Moreover, some enzymes from the enzymatic hydrolysis step remain functional during the subsequent fermentation process, and these enzymes will be capable of processing at least parts of the 16 % of unconverted cellulose remaining after enzymatic hydrolysis into glucose. These findings agree with the past studies done by Shen and Agblevor [20], who suggested that the enzyme cost for complete hydrolysis (100 % conversion of cellulose to glucose) is prohibitive in achieving the economically viable commercialization of ethanol production.
Conclusion
WEx pretreatment and enzymatic hydrolysis at high SC were found to produce high glucose yield from corn stover even at low enzyme loadings. Experimental results showed that the most optimal and economically feasible condition for high yields of glucose (about 84 %) was obtained when corn stover was wet-exploded at 170 °C for 20 min with 5.5 bar oxygen and subsequently enzymatically hydrolyzed at 20 % SC and 15 mg EP/g glucan. Significant enzyme cost saving can be achieved by accepting a glucose yield of about 80 % compared to the full conversion of the glucan of corn stover.
Abbreviations
- WEx:
-
Wet explosion
- EP:
-
Enzyme protein
- SC:
-
Substrate concentration
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- HPLC:
-
High-performance liquid chromatography
- FPU:
-
Filter paper units
- ANOVA:
-
Analysis of variance
- SEM:
-
Scanning electron microscopy
- GY:
-
Glucose yield
References
Banerjee G, Scott-Craig J, Walton J (2010) Improving enzymes for biomass conversion: a basic research perspective. Bioenergy Res 3:82–92
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE (2008) How biotech can transform biofuels. Nat Biotechnol 26:169–172
Kristensen J, Felby C, Jorgensen H (2009) Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol Biofuels 2:11
Kaar WE, Holtzapple MT (2000) Using lime pretreatment to facilitate the enzymic hydrolysis of corn stover. Biomass Bioenergy 18:189–199
Kim S, Holtzapple MT (2005) Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol 96:1994–2006
Kim TH, Lee YY (2005) Pretreatment of corn stover by soaking in aqueous ammonia. Appl Biochem Biotechnol 121:1119–1131
Bura R, Chandra R, Saddler J (2009) Influence of xylan on the enzymatic hydrolysis of steam-pretreated corn stover and hybrid poplar. Biotechnol Progr 25:315–322
Zhu JY, Zhu W, Obryan P, Dien B, Tian S, Gleisner R, Pan XJ (2010) Ethanol production from SPORL-pretreated lodgepole pine: preliminary evaluation of mass balance and process energy efficiency. Appl Microbiol Biotechnol 86:1355–1365
Yang J, Zhang XP, Yong QA, Yu SY (2011) Three-stage enzymatic hydrolysis of steam-exploded corn stover at high substrate concentration. Bioresour Technol 102:4905–4908
Ahring BK, Munck J (2009) Method for treating biomass and organic waste with the purpose of generating desired biologically based products. US Patent 0178671
Ahring BK, Jensen K, Nielsen P, Bjerre AB, Schmidt AS (1996) Pretreatment of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic bacteria. Bioresour Technol 58:107–113
Adney B, Baker J (2008) Measurement of cellulase activities. National Renewable Energy Laboratory, Golden
Rana D, Rana V, Ahring BK (2012) Producing high sugar concentrations from loblolly pine using wet explosion pretreatment. Bioresour Technol 121:61–67
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, Golden
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY, Mitchinson C, Saddler JN (2009) Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol Prog 25:333–339
Ingesson H, Zacchi G, Yang B, Esteghlalian AR, Saddler JN (2001) The effect of shaking regime on the rate and extent of enzymatic hydrolysis of cellulose. J Biotechnol 88:177–182
Wen ZY, Liao W, Chen SL (2004) Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour Technol 91:31–39
He X, Miao YL, Jiang XJ, Xu ZD, Ouyang PK (2010) Enhancing the enzymatic hydrolysis of corn stover by an integrated wet-milling and alkali pretreatment. Appl Biochem Biotechnol 162:2449–2457
Varga E, Szengyel Z, Reczey K (2002) Chemical pretreatments of corn stover for enhancing enzymatic digestibility. Appl Biochem Biotechnol 98:73–87
Shen J, Agblevor FA (2008) Optimization of enzyme loading and hydrolytic time in the hydrolysis of mixtures of cotton gin waste and recycled paper sludge for the maximum profit rate. Biochem Eng J 41:241–250
Acknowledgments
The authors gratefully acknowledge the financial support from the National Advanced Biofuels Consortium (NABC) and Department of Energy, USA (grant no. ZFT04064401). The authors would like to thank the Franceschi Microscopy and Imaging Center at Washington State University for SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rana, V., Rana, D. & Ahring, B.K. Process Modeling of Enzymatic Hydrolysis of Wet-Exploded Corn Stover. Bioenerg. Res. 7, 450–459 (2014). https://doi.org/10.1007/s12155-013-9384-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9384-6