Abstract
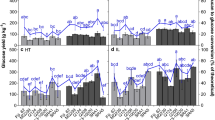
At the core of cellulosic ethanol research are innovations leading to reductions in the chemical and energetic stringency of thermochemical pretreatments and enzymatic saccharification. In this study, key compositional features of maize cell walls influencing the enzymatic conversion of biomass into fermentable sugars were identified. Stem samples from eight contrasting genotypes were subjected to a series of thermal dilute-acid pretreatments of increasing severity and evaluated for glucose release after enzymatic saccharification. The biochemically diverse set of genotypes displayed significant differences in glucose yields at all processing conditions evaluated. The results revealed that mechanisms controlling biomass conversion efficiency vary in relation to pretreatment severity. At highly severe pretreatments, cellulose conversion efficiency was primarily influenced by the inherent efficacy of the thermochemical process, and maximum glucose yields were obtained from cellulosic feedstocks harboring the highest cellulose contents per dry gram of biomass. When mild dilute-acid pretreatments were applied, however, maximum bioconversion efficiency and glucose yields were observed for genotypes combining high stem cellulose contents, reduced cell wall lignin and highly substituted hemicelluloses. For the best-performing genotype, glucose yields under sub-optimal processing regimes were only 10 % lower than the genotype-set mean at the most stringent processing conditions evaluated, while furfural production was reduced by approximately 95 %. Our results ultimately established that cellulosic feedstocks with tailored cell wall compositions can help reduce the chemical and energetic intensity of pretreatments used in the industry and improve the commercial and environmental performance of biomass-to-ethanol conversion technologies.






Similar content being viewed by others
References
Schubert C (2006) Can biofuels finally take center stage? Nature 24(7):777–784
Wyman CE (2007) What is (and is not) vital to advancing cellulosic ethanol? Trends Biotechnol 25(4):153–157
Yang B, Wyman CE (2007) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin 2(1):26–40
Elander R, Dale B, Holtzapple M, Ladisch M, Lee YY, Mitchinson C, Saddler J, Wyman C (2009) Summary of findings from the Biomass Refining Consortium for Applied Fundamentals and Innovation (CAFI): corn stover pretreatment. Cellulose 16(4):649–659. doi:10.1007/s10570-009-9308-y
Mosier N, Wyman C, Dale B, Elander R, Lee Y, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315(5813):804–807
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VGH (2012) Novel enzymes for the degradation of cellulose. Biotechnology for Biofuels 5(45)
Weber C, Farwick A, Benisch F, Brat D, Dietz H, Subtil T, Boles E (2010) Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl Microbiol Biotechnol 87(4):1303–1315
Lynd LR, Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16(5):577
Olson DG, McBride JE, Joe Shaw A, Lynd LR (2012) Recent progress in consolidated bioprocessing (Energy biotechnology • Environmental biotechnology). Curr Opin Biotechnol 23(3):396–405
Zhang D, VanFossen A, Pagano R, Johnson J, Parker M, Pan S, Gray B, Hancock E, Hagen D, Lucero H, Shen B, Lessard P, Ely C, Moriarty M, Ekborg N, Bougri O, Samoylov V, Lazar G, Raab RM (2011) Consolidated pretreatment and hydrolysis of plant biomass expressing cell wall degrading enzymes. BioEnergy Res 4(4):276–286
Becker J, Boles E (2003) A modified Saccharomyces cerevisiae strain that consumes l-arabinose and produces ethanol. Appl Environ Microbiol 69(7):4144–4150
Bera A, Ho NY, Khan A, Sedlak M (2011) A genetic overhaul of Saccharomyces cerevisiae 424A(LNH-ST) to improve xylose fermentation. J Ind Microbiol Biotechnol 38(5):617–626
Sedlak M, Ho NY (2004) Production of ethanol from cellulosic biomass hydrolysates using genetically engineered saccharomyces yeast capable of cofermenting glucose and xylose. Appl Biochem Biotechnol 114(1–3):403–416
Aden A, Foust T (2009) Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of corn stover to ethanol. Cellulose 16(4):535–545
Huang HJ, Ramaswamy S, Al-Dajani W, Tschirner U, Cairncross RA (2009) Effect of biomass species and plant size on cellulosic ethanol: a comparative process and economic analysis. Biomass Bioenergy 33(2):234–246
Tao L, Aden A, Elander RT, Pallapolu VR, Lee Y, Garlock RJ, Balan V, Dale BE, Kim Y, Mosier NS (2011) Process and technoeconomic analysis of leading pretreatment technologies for lignocellulosic ethanol production using switchgrass. Bioresour Technol 102(24):11105–11114
Chen F, Dixon RA (2007) Lignin modif improves fermentable sugar yields biofuel prod 25(7):759–761
Dien B, Sarath G, Pedersen J, Sattler S, Chen H, Funnell-Harris D, Nichols N, Cotta M (2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. BioEnergy Res 2(3):153–164
Fornalé S, Capellades M, Encina A, Wang K, Irar S, Lapierre C, Ruel K, Joseleau J-P, Berenguer J, Puigdomènech P, Rigau J, Caparrós-Ruiz D (2012) Altered lignin biosynthesis improves cellulosic bioethanol production in transgenic maize plants down-regulated for cinnamyl alcohol dehydrogenase. Mol Plant 5(4):817–830
Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M, Chen F, Foston M, Ragauskas A, Bouton J, Dixon RA, Wang Z-Y (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc Natl Acad Sci 108(9):3803–3808
Jung JH, Fouad WM, Vermerris W, Gallo M, Altpeter F (2012) RNAi suppression of lignin biosynthesis in sugarcane reduces recalcitrance for biofuel production from lignocellulosic biomass. Plant Biotechnol J 10(9):1067–1076
Lorenzana RE, Lewis MF, Jung HJG, Bernardo R (2010) Quantitative trait loci and trait correlations for maize stover cell wall composition and glucose release for cellulosic ethanol. Crop Sci 50(2):541–555
Masarin F, Gurpilhares DB, Baffa DCF, Barbosa MHP, Carvalho W, Ferraz A, Milagres AMF (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin contents. Biotechnoly Biofuels 4:55
Saballos A, Vermerris W, Rivera L, Ejeta G (2008) Allelic association, chemical characterization and saccharification properties of brown midrib mutants of sorghum (Sorghum bicolor (L.) Moench). BioEnergy Res 1(3–4):193–204
Sarath G, Dien B, Saathoff AJ, Vogel KP, Mitchell RB, Chen H (2011) Ethanol yields and cell wall properties in divergently bred switchgrass genotypes. Bioresour Technol 102(20):9579–9585
Lorenz AJ, Anex RP, Isci A, Coors JG, De Leon N, Weimer PJ (2009) Forage quality and composition measurements as predictors of ethanol yield from maize (Zea mays L.) stover. Biotechnoly for Biofuels 2(5)
Vermerris W, Saballos A, Ejeta G, Mosier NS, Ladisch MR, Carpita NC (2007) Molecular breeding to enhance ethanol production from corn and sorghum stover. Crop Sci 47(Supplement_3):S-142–S-153
Fontaine A-S, Bout S, Barrière Y, Vermerris W (2003) Variation in cell wall composition among forage maize (Zea mays L.) inbred lines and its impact on digestibility: analysis of neutral detergent fiber composition by pyrolysis-gas chromatography–mass spectrometry. J Agric Food Chem 51(27):8080–8087
Jung H-JG, Buxtono DR (1994) Forage quality variation among maize inbreds: relationships of cell-wall composition and in-vitro degradability for stem internodes. J Sci Food Agric 66(3):313–322
Dolstra O, Medema J An effective screening method for genetic improvement of cell-wall digestibility in forage maize. In: Proceedings 15th congress maize and sorghum section of Eucarpia, 1990. pp 4–8
Dolstra O, Medema J, De Jong A (1992) Genetic improvement of cell-wall digestibility in forage maize (Zea mays L.). I. Performance of inbred lines and related hybrids. Euphytica 65(3):187–194
Marvin HJ, Krechting CF, Van Loo EN, Snijders CH, Dolstra O (1995) Relationship between stalk cell wall digestibility and fibre composition in maize. J Sci Food Agric 69(2):215–221
Goering H, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications), vol 379. US Agricultural Research Service, Washington DC
Englyst HN, Cummings JH (1984) Simplified method for the measurement of total non-starch polysaccharides by gas–liquid chromatography of constituent sugars as alditol acetates. Analyst 109(7):937–942
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96(18):1967–1977
Pedersen M, Meyer AS (2010) Lignocellulose pretreatment severity—relating pH to biomatrix opening. New Biotechnol 27(6):739–750
Kabel MA, Bos G, Zeevalking J, Voragen AGJ, Schols HA (2007) Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour Technol 98(10):2034–2042
Selig M, Weiss N, Ji Y (1996) Enzymatic saccharification of lignocellulosic biomass; LAP-009 NREL analytical procedure. National Renewable Energy Laboratory, Golden
Lorenz A, Coors J, De Leon N, Wolfrum E, Hames B, Sluiter A, Weimer P (2009) Characterization, genetic variation, and combining ability of maize traits relevant to the production of cellulosic ethanol. Crop Sci 49(1):85–98
Schell DJ, Farmer J, Newman M, McMILLAN JD (2003) Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor. Appl Biochem Biotechnol 105(1):69–85
Esteghlalian A, Hashimoto AG, Fenske JJ, Penner MH (1997) Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour Technol 59(2):129–136
Yang R, Zhang C, Feng H, Yang W (2006) A kinetic study of xylan solubility and degradation during corncob steaming. Biosyst Eng 93(4):375–382
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23(6):1333–1339
Kabel MA, van den Borne H, Vincken JP, Voragen AGJ, Schols HA (2007) Structural differences of xylans affect their interaction with cellulose. Carbohydr Polym 69(1):94–105
Acknowledgments
We gratefully acknowledge Limagrain Nederland B.V. for providing us with specialized plant material and field trial support, and Genencor International B.V. for kindly supplying us with their cellulolytic enzyme cocktails. Within the framework of the Carbohydrate Competence Centre, this research has been financially supported by the European Union, the European Regional Development Fund, and the Northern Netherlands Provinces (Samenwerkingsverband Noord-Nederland), KOERS NOORD. We thank the reviewers and handling Co-Editor-in-Chief for helpful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres, A.F., van der Weijde, T., Dolstra, O. et al. Effect of Maize Biomass Composition on the Optimization of Dilute-Acid Pretreatments and Enzymatic Saccharification. Bioenerg. Res. 6, 1038–1051 (2013). https://doi.org/10.1007/s12155-013-9337-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9337-0