Abstract
Objective
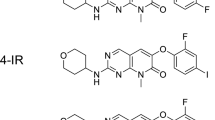
p38α, a member of the mitogen-activated protein kinase superfamily, is ubiquitously expressed in a variety of mammalian cells. Activated p38α induces inflammatory responses to external stimuli, suggesting that non-invasive detection of activated p38α would be valuable for diagnosing inflammatory diseases. For this purpose, we designed radiolabeled compounds [123I]2-IR and [123I]4-IR based on a potent p38α selective inhibitor R1487 for use with single photon emission computed tomography (SPECT). In this study, we used 125I instead of 123I due to its more usable radiochemical properties, synthesized [125I]2-IR and [125I]4-IR, and evaluated their effectiveness as activated p38α imaging probes.
Methods
[123I]2-IR and [123I]4-IR were designed by introduction of a 123I atom at the 2- or 4-ositions of the phenoxy ring, preserving the pyrimidinopyridone structure of R1487. We synthesized 2-IR and 4-IR via a 7-step process. The inhibitory potencies of 2-IR, 4-IR, and p38α inhibitors were measured using an ADP-Glo™ kinase assay system. Radioiodination of 2-IR and 4-IR was performed via an organotin-radioiodine exchange reaction using the corresponding tributyltin precursors. Biodistributions were evaluated by determining radioactivity in tissues of interest after intravenous administration of [125I]2-IR and [125I]4-IR in normal ddY mice and turpentine oil-induced inflammation model mice. In vivo inhibition study was also performed in inflammation model mice after intravenous administration of [125I]4-IR with pretreatment of p38α inhibitors.
Results
We synthesized 2-IR and 4-IR at total yields of 17.5% and 19.2%, respectively. 4-IR had higher p38α inhibitory potency than 2-IR; both compounds were significantly less potent than R1487. [125I]2-IR and [125I]4-IR were successfully obtained from tributyltin precursors with high radiochemical yield (> 65%), purity (> 97%), and molar activity (~ 81 GBq/µmol). [125I]4-IR showed high radioactivity accumulation in the inflamed tissue (7.0 ± 1.2%D/g), rapid delivery throughout the body, and rapid blood clearance, resulting in a high inflammation-to-blood ratio (6.2 ± 0.4) and a high inflammation-to-muscle ratio (5.2 ± 1.3) at 30 min, while [125I]2-IR showed low radioactivity accumulation in inflamed tissue over the experimental period. Further, radioactivity accumulation in inflamed tissue after [125I]4-IR administration was significantly decreased by pretreatment with selective inhibitors.
Conclusions
[123I]4-IR would be a promising imaging agent for detection of activated p38α.






Similar content being viewed by others
References
Cohen DM. Mitogen-activated protein kinase cascades and the signaling of hyperosmotic stress to immediate early genes. Comp Biochem Physiol A Physiol. 1997;117(3):291–9.
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2.
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13.
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–46.
Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, et al. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. Embo j. 1996;15(8):1914–23.
Cohen SB, Cheng TT, Chindalore V, Damjanov N, Burgos-Vargas R, Delora P, et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60(2):335–44.
Khalil MM, Tremoleda JL, Bayomy TB, Gsell W. Molecular SPECT imaging: an overview. Int J Mol Imaging. 2011;2011:796025.
Goldstein DM, Soth M, Gabriel T, Dewdney N, Kuglstatter A, Arzeno H, et al. Discovery of 6-(2,4-difluorophenoxy)-2-[3-hydroxy-1-(2-hydroxyethyl)propylamino]-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Pamapimod) and 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2H-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (R1487) as orally bioavailable and highly selective inhibitors of p38α mitogen-activated protein kinase. J Med Chem. 2011;54(7):2255–65.
OECD. Test No. 107: Partition Coefficient (n-octanol/water): Shake Flask Method. 1995.
RamaRao VV, Reddy GV, Maitraie D, Ravikanth S, Yadla R, Narsaiah B, Rao PS. One-pot synthesis of fluorine containing 3-cyano/ethoxycarbonyl-2-methyl-benzo[b]furans. Tetrahedron. 2004;60(52):12231–7.
Campbell RM, Anderson BD, Brooks NA, Brooks HB, Chan EM, De Dios A, et al. Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol Cancer Ther. 2014;13(2):364–74.
Zhang YY, Wu JW, Wang ZX. Mitogen-activated protein kinase (MAPK) phosphatase 3-mediated cross-talk between MAPKs ERK2 and p38alpha. J Biol Chem. 2011;286(18):16150–62.
Pellegrino D, Bonab AA, Dragotakes SC, Pitman JT, Mariani G, Carter EA. Inflammation and infection: imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med. 2005;46(9):1522–30.
Parida GK, Roy SG, Kumar R. FDG-PET/CT in skeletal muscle: pitfalls and pathologies. Semin Nucl Med. 2017;47(4):362–72.
Hirata M, Yao T, Fujimura S, Kanai Y, Yoshimoto M, Sato T, et al. Development of a p38α-selective radioactive probe for qualitative diagnosis of cancer using SPECT. Ann Nucl Med. 2019;33(5):333–43.
Patnaik A, Haluska P, Tolcher AW, Erlichman C, Papadopoulos KP, Lensing JL, et al. A first-in-human phase I study of the oral p38 MAPK inhibitor, Ralimetinib (LY2228820 Dimesylate), in patients with advanced cancer. Clin Cancer Res. 2016;22(5):1095–102.
Leelahavanichkul K, Amornphimoltham P, Molinolo AA, Basile JR, Koontongkaew S, Gutkind JS. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol Oncol. 2014;8(1):105–18.
Reustle A, Torzewski M. Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int J Mol Sci. 2018;19(12):3761.
Miura H, Kondo Y, Matsuda M, Aoki K. Cell-to-cell heterogeneity in p38-mediated cross-inhibition of JNK causes stochastic cell death. Cell Rep. 2018;24(10):2658–68.
Lin CL, Lee CH, Chen CM, Cheng CW, Chen PN, Ying TH, et al. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell Physiol Biochem. 2018;46(1):322–34.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
Grynberg K, Ma FY, Nikolic-Paterson DJ. The JNK signaling pathway in renal Fibrosis. Front Physiol. 2017;8:829.
Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, et al. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew Chem Int Ed Engl. 2014;53(30):7751–5.
Lim SSD, Jeon S, Kim Y, Kim H, Lee S, Cho H, Lee BC, Kim SE, Kim K, Lee E. Cobalt-catalyzed C-F bond borylation of aryl fluorides. Org Lett. 2018;20(22):7249–52.
Niwa TOH, Watanabe Y, Hosoya T. Ni/Cu-catalyzed defluoroborylation of fluoroarenes for diverse C-F Bond functionalizations. J Am Chem Soc. 2015;137(45):14313–8.
Airaksinen AJ. The radiopharmaceutical chemistry of Fluorine-18: next-generation fluorinations. Radiopharmaceutical Chemistry. 2019:297–310.
Makaravage KJBA, Mossine AV, Sanford MS, Scott PJH. Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org Lett. 2016;18(20):5440–3.
Acknowledgements
We are grateful for the technical assistance of Ms. Atsuko Takeguchi, Ms. Kaede Hanazono, Mr. Ryuji Kakisaka, Mr. Naofumi Yoshida, and Ms. Nami Nakai.
Funding
This work was supported in part by JSPS KAKENHI (19H03606).
Author information
Authors and Affiliations
Contributions
TH, MH, and TT conceptualized and designed the study. TH, NK, and MH performed the experiments and collected the data. TH, NK, MH, and TT interpreted the data. TH drafted the initial manuscript, which was critically reviewed by NK and TT. All authors approved the final manuscript and are accountable for all of the work.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interests. The funding bodies had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashimoto, T., Kondo, N., Hirata, M. et al. Development of radioiodinated pyrimidinopyridone derivatives as targeted imaging probes of activated p38α for single photon emission computed tomography. Ann Nucl Med 35, 1293–1304 (2021). https://doi.org/10.1007/s12149-021-01669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01669-6