Abstract
Objective
p38 mitogen-activated protein (MAP) kinase (p38α) has drawn attention as a new target molecule for the treatment and diagnosis of cancer, and its overexpression and activation have been reported in various types of cancer. In this study, a single photon emission computed tomography (SPECT) imaging probe of p38α was developed to noninvasively image p38α activity for effective qualitative diagnosis of cancer.
Methods
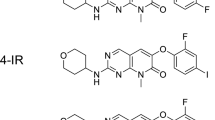
Pyrrolepyridine derivatives, m-YTM and p-YTM, were designed and synthesized based on the structure of the p38α-selective inhibitor. Radioactive iodine-labeled m-YTM, [125I]m-YTM, was synthesized because m-YTM greatly inhibited the phosphorylation of p38α upon examining the inhibitory effects of the compounds. After investigating the binding affinity of [125I]m-YTM to the recombinant p38α, a saturation binding experiment using activated p38α and inactive p38α was performed to determine the binding site. Uptake of [125I]m-YTM into various cancer cell lines was investigated, and the pharmacokinetics was evaluated using tumor-bearing mice.
Results
The inhibitory activity of m-YTM was approximately 13 times higher than that of SB203580, a p38α-selective inhibitor. The binding site of [125I]m-YTM was estimated to be the p38α activating site, similar to that of SB203580, because the [125I]m-YTM bound strongly to both activated p38α and inactive p38α. Various different cancer cells incorporated [125I]m-YTM; however, its accumulation was significantly reduced by treatment with SB203580. Pharmacokinetics study of [125I]m-YTM in B-16 tumor-bearing mice was examined which revealed high accumulation of radioactivity in tumor tissues. The ratios of radioactivity in the B-16 tumor to that in blood were 3.1 and 50 after 1 and 24 h, respectively. The ratio of radioactivity in the tumor to that in blood in the tumor-bearing mice generated using other cancer cell lines was also ≥ 1 at 1 h after the administration of the probe.
Conclusions
This study suggests that [123I]m-YTM has potential as a p38α imaging probe effective for various cancer types.










Similar content being viewed by others
References
Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4(1):82–9.
Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18(4):128–31.
Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays. 1996;18(7):567–77.
New L, Han J. The p38 MAP kinase pathway and its biological function. Trends Cardiovasc Med. 1998;8(5):220–8.
Lo U, Selvaraj V, Plane JM, Chechneva OV, Otsu K, Deng W. p38alpha (MAPK14) critically regulates the immunological response and the production of specific cytokines and chemokines in astrocytes. Sci Rep. 2014;4:7405.
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344(2):174–9.
Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, et al. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79(2):162–73.
Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99(13):1685–91.
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86(6):692–9.
Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, et al. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20(12):4506–14.
Walton KM, DiRocco R, Bartlett BA, Koury E, Marcy VR, Jarvis B, et al. Activation of p38MAPK in microglia after ischemia. J Neurochem. 1998;70(4):1764–7.
Barancik M, Htun P, Strohm C, Kilian S, Schaper W. Inhibition of the cardiac p38-MAPK pathway by SB203580 delays ischemic cell death. J Cardiovasc Pharmacol. 2000;35(3):474–83.
Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274(10):6272–9.
Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93(18):9641–4.
Han Q, Leng J, Bian D, Mahanivong C, Carpenter KA, Pan ZK, et al. Rac1-MKK3-p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem. 2002;277(50):48379–85.
Huang S, New L, Pan Z, Han J, Nemerow GR. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem. 2000;275(16):12266–72.
Igea A, Nebreda AR. The stress kinase p38alpha as a target for cancer therapy. Cancer Res. 2015;75(19):3997–4002.
Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4(9–10):342–59.
Henry JR, Rupert KC, Dodd JH, Turchi IJ, Wadsworth SA, Cavender DE, et al. Potent inhibitors of the MAP kinase p38. Bioorg Med Chem Lett. 1998;8(23):3335–40.
Markees DG, Dewey VC, Kidder GW. The synthesis and biological activity of substituted 2,6-diaminopyridines. J Med Chem. 1968;11(1):126–9.
Henry JR, Rupert KC, Dodd JH, Turchi IJ, Wadsworth SA, Cavender DE, et al. 6-Amino-2-(4-fluorophenyl)-4-methoxy-3- (4-pyridyl)-1H-pyrrolo[2, 3-b]pyridine (RWJ 68354): a potent and selective p38 kinase inhibitor. J Med Chem. 1998;41(22):4196–8.
Wilson KP, McCaffrey PG, Hsiao K, Pazhanisamy S, Galullo V, Bemis GW, et al. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem Biol. 1997;4(6):423–31.
Henry JR, Dodd JH. Synthesis of RWJ 68354: a potent inhibitor of the MAP kinase p38. Tetrahedron Lett. 1998;39(48):8763–4.
Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7(9):353–61.
Moriguchi T, Gotoh Y, Nishida E. Roles of the MAP kinase cascade in vertebrates. Adv Pharmacol. 1996;36:121–37.
Han H, Wang H, Long H, Nattel S, Wang Z. Oxidative preconditioning and apoptosis in L-cells. Roles of protein kinase B and mitogen-activated protein kinases. J Biol Chem. 2001;276(28):26357–64.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–31.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 12770512). There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirata, M., Yao, T., Fujimura, S. et al. Development of a p38α-selective radioactive probe for qualitative diagnosis of cancer using SPECT. Ann Nucl Med 33, 333–343 (2019). https://doi.org/10.1007/s12149-019-01341-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01341-0