Abstract
Objective
Aging decreases dopamine transporter (DAT) availability of striatum both in humans and rodents. We aimed to investigate the relationship of DAT availabilities from ventral striatum, caudate nucleus, and putamen with aging in healthy subjects.
Methods
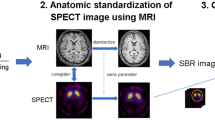
123I-FP-CIT single photon emission computed tomography (SPECT) was performed in all subjects. Specific binding of 123I-FP-CIT regarding DAT was calculated using a volume-of-interest-based analysis of ventral striatum, caudate nucleus, putamen. The cerebellum was chosen as a reference region. Specific binding ratios (SBRs) were calculated as follows: SBR = (target– cerebellum)/cerebellum.
Results
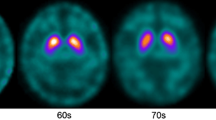
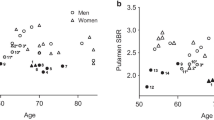
A total of 166 healthy subjects (109 males and 57 females) were included in this study. SBRs of ventral striatum, caudate nucleus, and putamen were negatively correlated with age. In young males, SBRs of ventral striatum and putamen were not correlated with aging. However, SBRs of caudate nucleus showed the trend toward negative correlation with age in the young group. In old males, SBR of caudate nucleus was negatively correlated with age and SBR of ventral striatum showed a trend toward negative correlation with age. Slopes of regression lines were not significantly different according to age groups in ventral striatum, caudate nucleus, or putamen. SBRs of ventral striatum, caudate nucleus, and putamen were negatively correlated with age in young females, but not in old females. Interestingly, slopes of regression line were significantly different between young and old females in ventral striatum, caudate nucleus, and putamen.
Conclusions
We have shown that slopes of regression lines of DAT availabilities and age were significantly different between young and old subjects in females, not in males. Therefore, sex has an impact on aging-related decline of striatal DAT availability.



Similar content being viewed by others
Change history
05 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12149-023-01841-0
References
Bajaj S, Alkozei A, Dailey NS, Killgore WDS. Brain aging: uncovering cortical characteristics of healthy aging in young adults. Front Aging Neurosci. 2017;9:412.
Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60(7):989–94.
Peters R. Ageing and the brain. Postgrad Med J. 2006;82(964):84–8.
Baez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233.
Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol. 2003;24(9):1849–56.
Fisone G, Hakansson K, Borgkvist A, Santini E. Signaling in the basal ganglia: postsynaptic and presynaptic mechanisms. Physiol Behav. 2007;92(1–2):8–14.
Caminero F, Cascella M. Neuroanatomy, Mesencephalon Midbrain. StatPearls. Treasure Island (FL). 2020.
Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89.
Park E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J Nucl Med Technol. 2012;40(4):222–8.
Ninerola-Baizan A, Rojas S, Roe-Vellve N, Lomena F, Ros D, Pavia J. Dopamine transporter imaging in the aged rat: a [(1)(2)(3)I]FP-CIT SPECT study. Nucl Med Biol. 2015;42(4):395–8.
Shingai Y, Tateno A, Arakawa R, Sakayori T, Kim W, Suzuki H, et al. Age-related decline in dopamine transporter in human brain using PET with a new radioligand [(1)(8)F]FE-PE2I. Ann Nucl Med. 2014;28(3):220–6.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213–27.
Yamamoto H, Arimura S, Nakanishi A, Shimo Y, Motoi Y, Ishiguro K, et al. Age-related effects and gender differences in Japanese healthy controls for [123I] FP-CIT SPECT. Ann Nucl Med. 2017;31(5):407–12.
Roberts G, Lloyd JJ, Petrides GS, Donaghy PC, Kane JPM, Durcan R, et al. 123I-FP-CIT striatal binding ratios do not decrease significantly with age in older adults. Ann Nucl Med. 2019;33(6):434–43.
Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629–35.
Garcia-Gomez FJ, Garcia-Solis D, Luis-Simon FJ, Marin-Oyaga VA, Carrillo F, Mir P, et al. Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane. Rev Esp Med Nucl Imagen Mol. 2013;32(6):350–6.
Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54(1):264–77.
Ryding E, Lindstrom M, Bradvik B, Grabowski M, Bosson P, Traskman-Bendz L, et al. A new model for separation between brain dopamine and serotonin transporters in 123I-beta-CIT SPECT measurements: normal values and sex and age dependence. Eur J Nucl Med Mol Imaging. 2004;31(8):1114–8.
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, et al. Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. J Nucl Med. 1995;36(7):1175–81.
Matsuda H, Murata M, Mukai Y, Sako K, Ono H, Toyama H, et al. Japanese multicenter database of healthy controls for [123I]FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2018;45(8):1405–16.
Eusebio A, Azulay JP, Ceccaldi M, Girard N, Mundler O, Guedj E. Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur J Nucl Med Mol Imaging. 2012;39(11):1778–833.
Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27(7):867–9.
Nobili F, Naseri M, De Carli F, Asenbaum S, Booij J, Darcourt J, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2013;40(4):565–73.
Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–9.
Bosse R, Rivest R, Di Paolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res. 1997;46(1–2):343–6.
Raghava N, Das BC, Ray SK. Neuroprotective effects of estrogen in CNS injuries: insights from animal models. Neurosci Neuroecon. 2017;6:15–29.
Labandeira-Garcia JL, Rodriguez-Perez AI, Valenzuela R, Costa-Besada MA, Guerra MJ. Menopause and Parkinson’s disease. Interaction between estrogens and brain renin-angiotensin system in dopaminergic degeneration. Front Neuroendocrinol. 2016;43:44–59.
Lee YH, Cha J, Chung SJ, Yoo HS, Sohn YH, Ye BS, et al. Beneficial effect of estrogen on nigrostriatal dopaminergic neurons in drug-naive postmenopausal Parkinson’s disease. Sci Rep. 2019;9(1):10531.
Mozley PD, Acton PD, Barraclough ED, Plossl K, Gur RC, Alavi A, et al. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999;40(11):1812–7.
Thomsen G, Knudsen GM, Jensen PS, Ziebell M, Holst KK, Asenbaum S, et al. No difference in striatal dopamine transporter availability between active smokers, ex-smokers and non-smokers using [123I]FP-CIT (DaTSCAN) and SPECT. EJNMMI Res. 2013;3(1):39.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301.
Tullo S, Patel R, Devenyi GA, Salaciak A, Bedford SA, Farzin S, et al. MR-based age-related effects on the striatum, globus pallidus, and thalamus in healthy individuals across the adult lifespan. Hum Brain Mapp. 2019;40(18):5269–88.
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10(1):36–433.
Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202.
Rubens R, Vermeulen A. Plasma testosterone levels in women in normal and pathological conditions. Eur J Obstet Gynecol. 1971;1(6):207–18.
Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):672–7.
Acknowledgements
PPMI is sponsored and partially funded by The Michael J. Fox Foundation for Parkinson’s Research (MJFF). Other funding partners include a consortium of industry players, non-profit organizations and private individuals; abbvie, Allergan, amathus therapeutics, Avid aradiopharmaceuticals, Biogen, BioLegend, Bristol-Myers Squibb, Celgene, DENALI, GE Healthcare, Genentech, GlaxoSmithKline, GOLUB CAPITAL, Handl Therapeutics, insitro, Janssen neuroscience, Lilly, Lundbeck, Merck, MSD, Pfizer, Piramal, Prevail therapeutics, Roche, SANOFI GENZYME, SERVIER, Takeda, TEVA, ucb, verily, Voyager therapeutics.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, S., Nam, HY., Lee, M.J. et al. Effect of sex on aging-related decline of dopamine transporter in healthy subjects. Ann Nucl Med 35, 76–82 (2021). https://doi.org/10.1007/s12149-020-01538-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01538-8