Abstract
Objective
I-123-2β-Carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane (FP-CIT) imaging is an established biomarker used in the diagnosis of Lewy body disease. Images are often reported with the aid of striatal binding ratios (SBRs), comparing uptake to a normal database via Z scores. It is well known that SBRs are age dependent. However, previous studies cover wide age ranges between 20 and 80 years, rather than focusing on older adults. Typically a linear relationship is reported, but some authors have suggested that SBRs do not decline as rapidly in old age. Commercial software packages usually adjust the SBR Z score to attempt to compensate for age-related decline, but the model used varies. Ensuring age correction is appropriate for older adults is important, given that the majority of patients referred for FP-CIT scans are aged over 60 years. We examined the relationship of SBR with age in older adults and the effect of age correction using research scans from 123 adults over 60 years of age.
Methods
Twenty-nine healthy older adults and twenty-three with MCI due to Alzheimer’s disease were included as controls, i.e. individuals with no evidence of Lewy body disease. Their ages ranged from 60 to 92 years (mean 76; SD 7.9). SBRs and Z scores were calculated using BRASS (Hermes Medical) and DaTQUANT (GE Healthcare). SBRs were plotted against age and linear mixed effect models applied. We tested the effect of removing age correction in BRASS using an independent dataset of 71 older adults with dementia or mild cognitive impairment.
Results
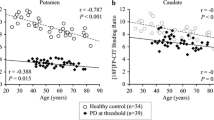
The slopes of the linear fits between SBR and age per year were − 0.007 (p = 0.30) with BRASS and − 0.004 (p = 0.35) with DaTQUANT. The slopes are smaller than reported in the literature and show no statistically significant difference from zero. Switching age correction off in BRASS in the test subjects reduced Z scores by approximately 1 standard deviation at 80 years of age.
Conclusion
We found no statistically significant age-related decline in SBR in adults over 60 years of age without Lewy body disease. Commercial software packages that apply a fixed rate of age correction may be overcorrecting for age in older adults, which could contribute to misdiagnosis.



Similar content being viewed by others
References
McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100.
Koch W, et al. Clinical testing of an optimized software solution for an automated, observer independent evaluation of dopamine transporter SPECT studies. J Nucl Med. 2005;46(7):10.
Calvini P, et al. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34(8):1240–53.
Nobili F, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2013;40(4):565–73.
Pencharz DR, et al. Automated quantification with BRASS reduces equivocal reporting of DaTSCAN (123I-FP-CIT) SPECT studies. Nucl Med Rev Cent East Eur. 2014;17(2):65–9.
Varrone A, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213–27 (15).
Booij J, et al. Diagnostic performance of the visual reading of (123)I-Ioflupane SPECT images with or without quantification in patients with movement disorders or dementia. J Nucl Med. 2017;58(11):1821–6.
Karrer TM, et al. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57:36–46.
van Dyck CH, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10(1):36–43.
Matsuda H, et al. Japanese multicenter database of healthy controls for [(123)I]FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2018;45(8):1405–16.
Koch W, et al. Extended studies of the striatal uptake of 99mTc-NC100697 in healthy volunteers. J Nucl Med. 2007;48(1):27–34.
Koch W, et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41(10):1938–46.
The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629–35.
Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
Thomas AJ, et al., Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2018;49:1–7.
Donaghy PC, et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med. 2018;48(14):2384–90.
Thomas AJ, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017;88(3):276–83.
McKeith I, et al., Sensitivity and specificity of dopamine transporter imaging with (123)I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6:305–13.
McKeith I, et al. Predictive accuracy of clinical diagnostic criteria for dementia with Lewy bodies: a prospective neuropathological validation study. Neurology. 2000;54(54):1050–8.
Markesbery WR, et al. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68(7):816–22.
Kane JPM, et al. 123I-MIBG scintigraphy utility and cut-off value in a clinically representative dementia cohort. Parkinsonism Relat Disord. 2019. https://doi.org/10.1016/j.parkreldis.2019.01.024.
Tossici-Bolt L, et al. Calibration of gamma camera systems for a multicentre European I-123-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging. 2011;38(8):1529–40.
Akahoshi M, et al. Attenuation and scatter correction in I-123 FP-CIT SPECT do not affect the clinical diagnosis of dopaminergic system neurodegeneration. Medicine (Baltimore). 2017;96(45):e8484.
Dickson JC, Tossici-Bolt L, Sera T, Booij J, Ziebell M, Morbelli S, Assenbaum-Nan S, Vander Borght T, Pagani M, Kapucu OL, Hesse S, The impact of reconstruction and scanner characterisation on the diagnostic capability of a normal database for [(123)I]FP-CIT SPECT imaging. Ejnmmi Res. 2017;7(1):10.
Pinheiro JC, Bates DM, Mixed-Effects Models in S and S-PLUS. New York: Springer Science & Business Media; 2000. p. 528
Yamamoto H, et al. Age-related effects and gender differences in Japanese healthy controls for [(123)I] FP-CIT SPECT. Ann Nucl Med. 2017;31(5):407–12.
Tossici-Bolt L, et al. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33(12):1491–9.
Acknowledgements
The authors would like to thank Miss Helen Kain, Research Support Secretary (Newcastle University Institute of Neuroscience) and our colleagues in the Nuclear Medicine department at the Newcastle Royal Victoria Infirmary. We thank the Alzheimer’s Society for supporting the Fellowship of the lead author and the NIHR and Alzheimer’s Research UK for supporting the clinical research studies used in this project. We are grateful to Liz Clarke (GE Healthcare) and Helena McMeekin (Hermes Medical) for providing information on DaTQUANT and BRASS, respectively.
Funding
The lead author is receiving full-time support from the Alzheimer’s Society via a Clinical Training Fellowship. The clinical studies used in this project were funded by Alzheimer’s Research UK and the Newcastle National Institute for Health Research (NIHR) Biomedical Research Centre, hosted by Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
123I-FP-CIT radiopharmaceutical and the DaTQUANT software package were provided by GE Healthcare. We have no other conflicts of interest relevant to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberts, G., Lloyd, J.J., Petrides, G.S. et al. 123I-FP-CIT striatal binding ratios do not decrease significantly with age in older adults. Ann Nucl Med 33, 434–443 (2019). https://doi.org/10.1007/s12149-019-01352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01352-x