Abstract
Objective
Exact classifying between malignant and benign tumors in the parotid gland is important because the cancer has relatively poor prognosis. There have been several studies that F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) can differentiate between malignant and benign parotid gland tumors. However, the role of FDG PET is still controversial because many benign parotid gland tumors, such as Warthin’s tumor and pleomorphic adenoma, show high FDG uptake. We hypothesized that metabolic heterogeneity would differentiate malignant parotid tumors because tumoral heterogeneity is an important characteristic in the malignancies.
Methods
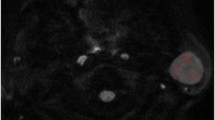
From January 2010 to April 2015, we retrospectively reviewed the 46 patients who showed FDG uptake at the parotid gland. To differentiate malignant parotid gland tumors, we obtained maximum SUV and mean SUV. Metabolic tumor volume and total lesion glycolysis were measured as metabolic volumetric parameters. We also included heterogeneity parameters of FDG PET such as heterogeneity factor (HF) and the coefficient of variation for all patients.
Results
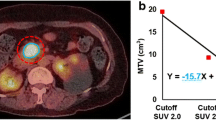
There was significant difference of HF between malignant (−0.30 ± 0.25; range −0.937 to −0.084) and benign parotid gland tumors (−0.06 ± 0.05; range −0.291 to −0.012; p < 0.0001). In receiver operating characteristic analysis, when ≤−0.084 was used as the cut-off value for HF, the sensitivity and specificity were 100 % (95 % CI 81.5–100) and 89.2 % (95 % CI 71.8–97.7), respectively. HF showed the highest area under the curve of 0.947 among the parameters. In logistic regression analysis, the HF was the most powerful factor for differentiation of the parotid gland tumors (p = 0.002).
Conclusions
Our results suggest that HF can be utilized as a reliable and non-invasive method for differentiation of malignant and benign parotid gland tumors.





Similar content being viewed by others
References
Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229–40.
Ho K, Lin H, Ann DK, Chu PG, Yen Y. An overview of the rare parotid gland cancer. Head Neck Oncol. 2011;3:40.
Lima RA, Tavares MR, Dias FL, Kligerman J, Nascimento MF, Barbosa MM, et al. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg. 2005;133(5):702–8.
Uchida Y, Minoshima S, Kawata T, Motoori K, Nakano K, Kazama T, et al. Diagnostic value of FDG PET and salivary gland scintigraphy for parotid tumors. Clin Nucl Med. 2005;30(3):170–6.
Roh JL, Yeo NK, Kim JS, Lee JH, Cho KJ, Choi SH, et al. Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43(9):887–93.
Keyes JW Jr, Watson NE Jr, Williams DW 3rd, Greven KM, McGuirt WF. FDG PET in head and neck cancer. AJR Am J Roentgenol. 1997;169(6):1663–9.
Seo YL, Yoon DY, Baek S, Lim KJ, Yun EJ, Cho YK, et al. Incidental focal FDG uptake in the parotid glands on PET/CT in patients with head and neck malignancy. Eur Radiol. 2015;25(1):171–7.
Hadiprodjo D, Ryan T, Truong MT, Mercier G, Subramaniam RM. Parotid gland tumors: preliminary data for the value of FDG PET/CT diagnostic parameters. AJR Am J Roentgenol. 2012;198(2):W185–90.
Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, et al. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46(4):675–82.
Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62(2):545–53.
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–15.
Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9(4):539–49.
Budiawan H, Cheon GJ, Im HJ, Lee SJ, Paeng JC, Kang KW, et al. Heterogeneity analysis of (18)F-FDG uptake in differentiating between metastatic and inflammatory lymph nodes in adenocarcinoma of the lung: comparison with other parameters and its application in a clinical setting. Nucl Med Mol Imaging. 2013;47(4):232–41.
Huang B, Chan T, Kwong DL, Chan WK, Khong PL. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentgenol. 2012;199(1):169–74.
Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14(16):5236–41.
Kang SR, Song HC, Byun BH, Oh JR, Kim HS, Hong SP, et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Nucl Med Mol Imaging. 2014;48(1):16–25.
Eary JF, O’Sullivan F, O’Sullivan J, Conrad EU. Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med. 2008;49(12):1973–9.
Son SH, Kim DH, Hong CM, Kim CY, Jeong SY, Lee SW, et al. Prognostic implication of intratumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer. 2014;14:585.
Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005;234(3):879–85.
Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49(2):201–8.
Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15(18):5861–8.
Roh JL, Kim JS, Kang BC, Cho KJ, Lee SW, Kim SB, et al. Clinical significance of pretreatment metabolic tumor volume and total lesion glycolysis in hypopharyngeal squamous cell carcinomas. J Surg Oncol. 2014;110(7):869–75.
Kwon SH, Yoon JK, An YS, Shin YS, Kim CH, Lee DH, et al. Prognostic significance of the intratumoral heterogeneity of (18) F-FDG uptake in oral cavity cancer. J Surg Oncol. 2014;110(6):702–6.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–45.
Krak NC, Boellaard R, Hoekstra OS, Twisk JW, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32(3):294–301.
van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38(9):1636–47.
Gerlee P, Anderson AR. An evolutionary hybrid cellular automaton model of solid tumour growth. J Theor Biol. 2007;246(4):583–603.
Jin GQ, Su DK, Xie D, Zhao W, Liu LD, Zhu XN. Distinguishing benign from malignant parotid gland tumours: low-dose multi-phasic CT protocol with 5-minute delay. Eur Radiol. 2011;21(8):1692–8.
Stein AP, Britt CJ, Saha S, McCulloch TM, Wieland AM, Harari PM, et al. Patient and tumor characteristics predictive of primary parotid gland malignancy: a 20-year experience at the University of Wisconsin. Am J Otolaryngol. 2015;36(3):429–34.
Kovacevic DO, Fabijanic I. Sonographic diagnosis of parotid gland lesions: correlation with the results of sonographically guided fine-needle aspiration biopsy. J Clin Ultrasound. 2010;38(6):294–8.
Acknowledgments
This work was supported by a 2-year research Grant of Pusan National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, B.S., Kim, SJ. & Pak, K. Diagnostic value of metabolic heterogeneity as a reliable parameter for differentiating malignant parotid gland tumors. Ann Nucl Med 30, 346–354 (2016). https://doi.org/10.1007/s12149-016-1068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1068-9