Abstract
Objective
6-[18F]Fluoro-l-DOPA (FDOPA) is a radiopharmaceutical valuable for assessing the presynaptic dopaminergic function when used with positron emission tomography (PET). More specifically, the striatal-to-occipital ratio (SOR) of FDOPA uptake images has been extensively used as a quantitative parameter in these PET studies. Our aim was to develop an easy, automated method capable of performing objective analysis of SOR in FDOPA PET images of Parkinson’s disease (PD) patients.
Methods
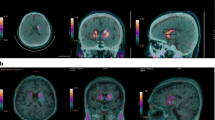
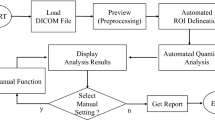
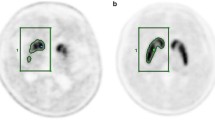
Brain images from FDOPA PET studies of 21 patients with PD and 6 healthy subjects were included in our automated striatal analyses. Images of each individual were spatially normalized into an FDOPA template. Subsequently, the image slice with the highest level of basal ganglia activity was chosen among the series of normalized images. Also, the immediate preceding and following slices of the chosen image were then selected. Finally, the summation of these three images was used to quantify and calculate the SOR values. The results obtained by automated analysis were compared with manual analysis by a trained and experienced image processing technologist.
Results
The SOR values obtained from the automated analysis had a good agreement and high correlation with manual analysis. The differences in caudate, putamen, and striatum were −0.023, −0.029, and −0.025, respectively; correlation coefficients 0.961, 0.957, and 0.972, respectively.
Conclusions
We have successfully developed a method for automated striatal uptake analysis of FDOPA PET images. There was no significant difference between the SOR values obtained from this method and using manual analysis. Yet it is an unbiased time-saving and cost-effective program and easy to implement on a personal computer.






Similar content being viewed by others
References
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80.
Garnett ES, Firnau G, Nahmias C. Dopamine visualized in the basal ganglia of living man. Nature. 1983;305:137–8.
Nahmias C, Garnett ES, Firnau G, Lang A. Striatal dopamine distribution in parkinsonian patients during life. J Neurol Sci. 1985;69:223–30.
Leenders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, et al. Brain dopamine metabolism in patients with Parkinson’s disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry. 1986;49:853–60.
Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119:585–91.
Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, Zanzi I, et al. Input functions for 6-[fluorine-18]fluorodopa quantitation in parkinsonism: comparative studies and clinical correlations. J Nucl Med. 1994;35:955–63.
Ishikawa T, Dhawan V, Chaly T, Margouleff C, Robeson W, Dahl JR, et al. Clinical significance of striatal DOPA decarboxylase activity in Parkinson’s disease. J Nucl Med. 1996;37:216–22.
Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Neumeyer J, et al. Comparative nigrostriatal dopaminergic imaging with iodine-123-βCIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–5.
Dhawan V, Ma Y, Pillai V, Spetsieris P, Chaly T, Belakhlef A, et al. Comparative analysis of striatal FDOPA uptake in Parkinson’s disease: ratio method versus graphical approach. J Nucl Med. 2002;43:1324–30.
Eshuis SA, Maguire RP, Leenders KL, Jonkman S, Jager PL. Comparison of FP-CIT SPECT with F-DOPA PET in patients with de novo and advanced Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2006;33:200–9.
Eshuis SA, Jager PL, Maguire RP, Jonkman S, Dierckx RA, Leenders KL. Direct comparison of FP-CIT SPECT and F-DOPA PET in patients with Parkinson’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2009;36:454–62.
Jokinen P, Helenius H, Rauhala E, Brück A, Eskola O, Rinne JO. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J Nucl Med. 2009;50:893–9.
Huang WS, Chiang YH, Lin JC, Chou YH, Cheng CY, Liu RS. Crossover study of 99mTc-TRODAT-1 SPECT and 18F-FDOPA PET in Parkinson’s disease. J Nucl Med. 2003;44:999–1005.
Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–89.
Ashburner J, Neelin P, Collins DL, Evans A, Friston KJ. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–52.
Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66.
Kao CH, Hsu WL, Xie HL, Lin MC, Lan WC, Chao HY. GMP production of [18F]FDOPA and issues concerning its quality analyses as in USP “Fluorodopa F 18 injection”. Ann Nucl Med. 2011;25:309–16.
Meyer JH, Gunn RN, Myers R, Grasby PM. Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage. 1999;9:545–53.
Gispert JD, Pascau J, Reig S, Martínez-Lázaro R, Molina V, García-Barreno P, et al. Influence of the normalization template on the outcome of statistical parametric mapping of PET scans. Neuroimage. 2003;19:601–12.
Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. Automated multi-modality image registration based on information theory. In: Bizais Y, Barillot C, Di Paolo R, editors. Information processing in medical imaging. Dordrecht: Kluwer Academic Publishers; 1995. p. 263–74.
Studholme C, Hill DLG, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit. 1999;32:71–86.
Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. In: Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference; 1993. p. 1813–7.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Habraken JB, Booij J, Slomka P, Sokole EB, van Royen EA. Quantification and visualization of defects of the functional dopaminergic system using an automatic algorithm. J Nucl Med. 1999;40:1091–7.
Radau PE, Linke R, Slomka PJ, Tatsch K. Optimization of automated quantification of 123I-IBZM uptake in the striatum applied to parkinsonism. J Nucl Med. 2000;41:220–7.
Buchert R, Berding G, Wilke F, Martin B, von Borczyskowski D, Mester J, et al. IBZM tool: a fully automated expert system for the evaluation of IBZM SPECT studies. Eur J Nucl Med Mol Imaging. 2006;33:1073–83.
Zubal IG, Early M, Yuan O, Jennings D, Marek K, Seibyl JP. Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson’s disease patients. J Nucl Med. 2007;48:857–64.
Mirzaei S, Zakavi R, Rodrigues M, Schwarzgruber T, Brücke T, Bakala J, et al. Fully automated 3D basal ganglia activity measurement in dopamine transporter scintigraphy (Spectalyzer). Ann Nucl Med. 2010;24:295–300.
Seibyl JP. Single-photon emission computed tomography and positron emission tomography evaluations of patients with central motor disorders. Semin Nucl Med. 2008;38:274–86.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–20.
Acknowledgments
The authors thank Dr. Shin-Yuan Chen and Ms. Li-Chuan Huang for providing the clinical information of PD patients and the MR imaging related parameters, respectively. This study was supported by a grant TCRD 99-27 from the Buddhist Tzu Chi General Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, IC., Lue, KH., Hsieh, HJ. et al. Automated striatal uptake analysis of 18F-FDOPA PET images applied to Parkinson’s disease patients. Ann Nucl Med 25, 796–803 (2011). https://doi.org/10.1007/s12149-011-0533-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-011-0533-8