Abstract
Objective
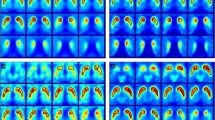
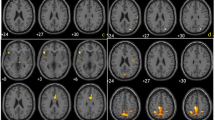
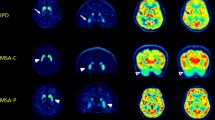
We applied a simple isocontour volume-of-interest (VOI) method to analyze the whole striatum in an F-18 FP-CIT PET image and to investigate the usefulness of the method in differentiating healthy subjects from idiopathic Parkinson’s disease (IPD) patients and the correlation of the value of functional volume parameters with the motor symptoms in patients with IPD.
Methods
Forty-three IPD patients and 23 age-matched healthy controls underwent F-18 FP-CIT PET. Using a dedicated workstation, VOIs for the whole striatum were drawn automatically with the gradient delineation method. The SUVmax, SUVmean, functional volume (FV), striatal volume activity (SVA), striatal-specific binding (SSB), and volume-specific uptake ratio (VSUR) were compared between the IPD patients and the normal subjects. In the IPD patients, the correlation between the clinical factor and the functional parameters was assessed.
Results
The SUVmax, SUVmean, FV, SVA, SSB, and VSUR were significantly lower in the IPD patients than in the normal subjects. In the receiver operating characteristic analysis, those parameters had significant and good-to-excellent accuracy. In the patients with IPD, a moderate negative correlation was revealed between the SUVmax and H&Y stage, the SUVmean and H&Y stage, SVA and H&Y stage, the VSUR and H&Y stage, the FV and bradykinesia, and the SVA and bradykinesia.
Conclusion
The functional volumetric analysis of the striatum based on simple isocontour VOI was a useful method of analyzing the F-18 FP-CIT PET image. Not only can it be easily applied in daily clinical practice, but it can also be used as a clinical parameter to discriminate IPD and to correlate it with the disease severity.



Similar content being viewed by others
References
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain. 2011;134:3146–66.
Meyer PT, Hellwig S. Update on SPECT and PET in parkinsonism—part 1: imaging for differential diagnosis. Curr Opin Neurol. 2014;27:390–7.
Ba F, Martin WW. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Park Relat Disord. 2015;21:87–94.
Benitez-Rivero S, Marin-Oyaga VA, Garcia-Solis D, Huertas-Fernandez I, Garcia-Gomez FJ, Jesus S, et al. Clinical features and 123I-FP-CIT SPECT imaging in vascular parkinsonism and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84:122–9.
Nissen T, Malek N, Grosset K, Newman E, Patterson J, Hadley D, et al. Baseline [123I] FP-CIT SPECT (DaTSCAN) severity correlates with medication use at 3 years in Parkinson’s disease. Acta Neurol Scand. 2014;129:204–8.
Huang W, Lee M, Lin J, Chen C, Yang Y, Lin S, et al. Usefulness of brain 99mTc-TRODAT-1 SPET for the evaluation of Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2004;31:155–61.
Song IU, Kim YD, Cho HJ, Chung SW, Chung YA. An FP-CIT PET comparison of the differences in dopaminergic neuronal loss between idiopathic Parkinson disease with dementia and without dementia. Alzheimer Dis Assoc Disord. 2013;27:51–5.
Kraemmer J, Kovacs GG, Perju-Dumbrava L, Pirker S, Traub-Weidinger T, Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29:1767–73.
Wang J, Zuo C, Jiang Y, Guan Y, Chen Z, Xiang J, et al. 18F-FP-CIT PET imaging and SPM analysis of dopamine transporters in Parkinson’s disease in various Hoehn & Yahr stages. J Neurol. 2007;254:185–90.
Jin S, Oh M, Oh SJ, Oh JS, Lee SJ, Chung SJ, et al. Differential diagnosis of parkinsonism using dual-phase F-18 FP-CIT PET imaging. Nucl Med Mol Imaging. 2013;47:44–51.
Eshuis S, Maguire R, Leenders K, Jonkman S, Jager P. Comparison of FP-CIT SPECT with F-DOPA PET in patients with de novo and advanced Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2006;33:200–9.
Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2013;84:1288–95.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I] FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40:213–27.
Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406.
Scherfler C, Nocker M. Dopamine transporter SPECT: How to remove subjectivity? Mov Disord. 2009;24:S721–4.
Kim Y, Im H, Paeng JC, Lee JS, Eo JS, Kim DH, et al. Validation of simple quantification methods for 18F-FP-CIT PET using automatic delineation of volumes of interest based on statistical probabilistic anatomical mapping and isocontour margin setting. Nucl Med Mol Imaging. 2012;46:254–60.
Jeong E, Oh SY, Pahk K, Lee C, Park K, Lee JS, et al. Feasibility of PET template-based analysis on F-18 FP-CIT PET in patients with de novo Parkinson’s disease. Nucl Med Mol Imaging. 2013;47:73–80.
Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39:1521–30.
Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12.
Faro A, Giordano D, Spampinato C, Ullo S, Stefano AD. Basal ganglia activity measurement by automatic 3-D striatum segmentation in SPECT images. IEEE Trans Instrum Meas. 2011;60:3269–80.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 2001;57(Suppl 3):S11–26.
Fahn S, Elton R. Members of the UPDRS development committee. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987. p. 153–63, 293–304.
Werner-Wasik M, Nelson AD, Choi W, Arai Y, Faulhaber PF, Kang P, et al. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys. 2012;82:1164–71.
Varrone A, Halldin C. Molecular imaging of the dopamine transporter. J Nucl Med. 2010;51:1331–4.
Benamer HT, Patterson J, Grosset DG, Booij J, De Bruin K, Van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. 2000;15:503–10.
Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18:S43–51.
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee G, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15:692–8.
Sridhar P, Mercier G, Tan J, Truong MT, Daly B, Subramaniam RM. FDG PET metabolic tumor volume segmentation and pathologic volume of primary human solid tumors. AJR Am J Roentgenol. 2014;202:1114–9.
Abedelahi A, Hasanzadeh H, Hadizadeh H, Joghataie MT. Morphometric and volumetric study of caudate and putamen nuclei in normal individuals by MRI: effect of normal aging, gender and hemispheric differences. Pol J Radiol. 2013;78:7–14.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133–40.
Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsoter N, Papp L, et al. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55:891–7.
Acknowledgments
This work was supported by the Busan Metropolitan City research fund (2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Jeong, Y.J., Son, H.J., Yoon, H.J. et al. Functional volumetric analysis of striatum using F-18 FP-CIT PET in patients with idiopathic Parkinson’s disease and normal subjects. Ann Nucl Med 30, 572–578 (2016). https://doi.org/10.1007/s12149-016-1096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1096-5