Abstract
Objective
The aim of this study was to evaluate the potential role of F-18-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) in assessing the chemotherapy response of osteosarcoma when compared with histologically assessed tumor necrosis.
Methods
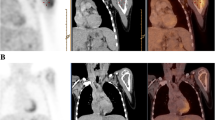
Fifteen patients were examined with whole-body FDG-PET prior to and following neoadjuvant therapy. The maximum standard uptake value (SUVmax) of tumor and tumor to background ratio (TBR) prior to and following chemotherapy was used for semiquantitative PET imaging analysis. The SUVmax of prechemotherapy and post-chemotherapy was recorded as SUV1 and SUV2. TBR1 and TBR2 represented prechemotherapy and post-chemotherapy TBR. TBR was calculated by drawing an identical region of interest over the tumor and the contralateral normal limb or pelvis. Tumor necrosis was classified according to Salzer-Kuntschik’s criteria.
Results
Eight patients with more than 90% tumor necrosis were classified as showing good responses and seven patients with less than 90% tumor necrosis as showing poor responses. SUV2/SUV1, TBR2/TBR1, and TBR2 were significantly correlated with the tumor necrosis degree (P < 0.01, P < 0.001, P < 0.001). TBR2/TBR1 were below 0.46 in all the patients with favorable responses, and higher than 0.49 in all the patients with unfavorable responses. However, it was difficult to distinguish good responses from poor responses by SUV2/SUV1.
Conclusions
FDG-PET is a promising tool to assess the chemotherapy response of osteosarcoma noninvasively. The TBR was better than SUVmax in evaluating the chemotherapy response in this study.
Similar content being viewed by others
References
Saeter G, Kloke O, Jelic S; ESMO Guidelines Task Force. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of osteosarcoma. Ann Oncol 2005;16Suppl 1:i71–i72.
Bielack S, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–790.
Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001;19:3203–3209.
Bruzzi JF, Truong M, Zinner R, Erasmus JJ, Sabloff B, Munden R. Short-term restaging of patients with non-small cell lung cancer receiving chemotherapy. J Thorac Oncol 2006;1:425–429.
Schwarz JD, Bader M, Jenicke L, Hemminger G, Janicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG-PET. J Nucl Med 2005;46:1144–1150.
Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 2005;16:1514–1523.
Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging 2004;31:5–12.
Levine EA, Farmer MR, Clark P, Mishra G, Ho C, Geisinger KR. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg 2006;243:472–478.
Feldman F, Heertum RV, Saxena C, Parisien M. 18FDG-PET applications for cartilage neoplasms. Skeletal Radiol 2005;34:367–374.
Györke T, Zajic T, Lange A, Schafer O, Moser E, Mako E, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun 2006;27:17–24.
Kneisl JS, Patt JC, Johnson JC, Zuger JH. Is PET useful in detecting occult nonpulmonary metastases in pediatric bone sarcomas? Clin Orthop Relat Res 2006;450:101–104.
Tateishi U, Yamaguchi U, Seki U, Terauchi T, Arai Y, Hasegawa T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue [18F]fluorodeoxyglucose sarcomas: a prospective evaluation by positron emission tomography. Eur J Nucl Med Mol Imaging 2006;33:683–691.
Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging 2004;31:189–195.
Zhang H, Yoshikawa K, Tamura K, Tomemori T, Sagou K, Tian M, et al. [(11)C] methionine positron emission tomography and survival in patients with bone and soft tissue sarcomas treated by carbon ion radiotherapy. Clin Cancer Res 2004;10:1764–1772.
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 1980;153:106–120.
Salzer-Kuntschik M, Delling G, Beron G, Sigmund R. Morphological grades of regression in osteosarcoma after polychemotherapy-study COSS 80. J Cancer Res Clin Oncol 1983;106Suppl:21–24.
Cullen JW, Jamroz BA, Stevens SL, Madsen W, Hinshaw I, Wilkins RM, et al. The value of serial arteriography in osteosarcoma: delivery of chemotherapy, determination of therapy duration, and prediction of necrosis. J Vasc Interv Radiol 2005;16:1107–1119.
Kunisada T, Ozaki T, Kawai A, Sugihara S, Taguchi K, Inoue H. Imaging assessment of the responses of osteosarcoma patients to preoperative chemotherapy:angiography compared with thallium-201 scintigraphy. Cancer 1999;86:949–956.
Dyke JP, Panicek DM, Healey JH, Meyers PA, Huvos AG, Schwartz LH, et al. Osteogenic and Ewing sarcomas: estimation of necrotic fraction during Induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology 2003;228:271–278.
Jones DN, McCowage GB, Sostman HD, Brizel DM, Layfield L, Charles HC, et al. Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med 1996;37:1438–1444.
Abella E, Dicarli M, Ravindranath Y, Kottamasu S, Muzik O, Decamilo D, et al. Positron emission tomography [PET] scanning with 18-fluorodeoxyglcose[FDG] correlates with histological response in children with osteosarcoma [OS] and Ewing’s sarcoma [ES]. Proc Annu Meet Am Soc Clin Oncol 1996;15:1457.
Schulte M, Brecht-Krauss D, Werner M, Hartwig E, Sarkar MR, Keppler P, et al. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med 1999;40:1637–1643.
Franzius C, Sciuk J, Brinkschmidt C, Jurgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med 2000;25:874–881.
Nair N, Ali A, Green AA, Lamonica G, Alibazoglu H, Alibazoglu B, et al. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging 2000;3:79–83.
Hawkins DS, Rajendran JG, Conrad EU III, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer 2002;94:3277–3284.
Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–724.
Hawkins DS, Schuteze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU III, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–8834.
Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU III, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005;103:339–348.
Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med 1993;34:355–359.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Z., Zhu, J., Tian, M. et al. Response of osteogenic sarcoma to neoadjuvant therapy: evaluated by 18F-FDG-PET. Ann Nucl Med 22, 475–480 (2008). https://doi.org/10.1007/s12149-008-0147-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0147-y