Abstract
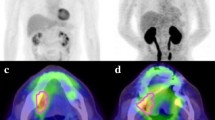
Increased expression of glucose transporter-1 (Glut-1) and glucose transporter-3 (Glut-3) has been reported in many human cancers. The mechanism of glucose entry into oral squamous cell carcinoma (OSCC) remains unclear. In this study we investigated, in untreated human OSCC, the relationship between tumour fluorine-18 fluoro-2-deoxy-d-glucose (FDG) accumulation and the expression of Glut-1 and Glut-3, as well as the association between the expression of Glut-1 and of Glut-3. All patients underwent FDG positron emission tomography (PET) pre-operatively. Standardised uptake values (SUVs) were used for evaluation of tumour FDG uptake. Final diagnoses were established by histology. Immunohistochemical staining results were evaluated according to the percentage (%) of positive area, intensity and staining score. Tumour sections were stained by immunohistochemistry for Glut-1 and Glut-3. Glut-1 immunostaining revealed that 18 (94.7%) of the 19 tumours stained positively, while Glut-3 immunostaining yielded positive findings for 16 (84.2%) tumours. Overall, a relatively low level of agreement (36.8%) in the staining score was observed between Glut-1 and Glut-3 expression. No relationship was found between the staining pattern and tumour differentiation or T grade classification in either Glut-1 or Glut-3 immunostaining. Furthermore, no relationship was found between increased FDG SUV and tumour differentiation, but the former did correlate with T grade. In conclusion, high FDG uptake values were seen in OSCC with overexpression of Glut-1 and Glut-3. However, no significant correlation was found between FDG SUV and Glut-1 or Glut-3 expression.

Similar content being viewed by others
References
Ak I, Stokkel MP, Pauwels EK. Positron emission tomography with 2-[18F]fluoro-2-deoxy-d-glucose in oncology. Part II. The clinical value in detecting and staging primary tumours. J Cancer Res Clin Oncol 2000; 126:560–574.
Warburg O, Posenser K, Negelein E. The metabolism of the carcinoma cell. In: The metabolism of tumors. New York: Richard R. Smith; 1931:129–169.
Isselbacher K. Sugar and amino acid transport by cells in culture—differences between normal and malignant cells. N Engl J Med 1972; 286:929–933.
Stokkel MP, Linthorst MF, Borm JJ, Taminiau AH, Pauwels EK. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J Cancer Res Clin Oncol 2002; 128:393–399.
Stuart Wood I, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003; 89:3–9.
Oehr P, Ruhlmann J, Biersack HJ. FDG-PET in clinical oncology: review of the literature and report of one institution’s experience. J Investig Med 1999; 47:452–461.
Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 1990; 170:223–230.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 1993; 34:1–6.
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated-FDG in breast carcinoma: correlation between Glut-1 expression and FDG uptake. J Nucl Med 1996; 37:1042–1047.
Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, Doi R, Hosotani R, Imamura M, Konishi J. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med 2002; 43:173–180.
Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol 2002; 29:443–453.
Mellanen P, Minn H, Grenman R, Harkonen P. Expression of glucose transporters in head-and-neck tumors. Int J Cancer 1994; 56:622–629.
Marom EM, Aloia TA, Moore MB, Hara M, Herndon JE 2nd, Harpole DH Jr, Goodman PC, Patz EF Jr. Correlation of FDG-PET imaging with Glut-1 and Glut-3 expression in early-stage non-small cell lung cancer. Lung Cancer 2001; 33:99–107.
Higashi T, Tamaki N, Honda T, Torizuka T, Kimura T, Inokuma T, Ohshio G, Hosotani R, Imamura M, Konishi J. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J Nucl Med 1997; 38:1337–1344.
UICC: TNM classification of malignant tumors, 4th edn. In: Hermanek P, Sobin LH, eds. Berlin Heidelberg New York: Springer, 1987.
Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 1986; 27:235–238.
Zhang H, Inoue T, Alyafei S, Tian M, Oriuchi N, Endo K. Tumour detectability in 2-dimensional and 3-dimensional positron emission tomography using the SET-2400 W: a phantom study. Nucl Med Commun 2001; 22:305–314.
Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res 1996; 56:1164–1167.
Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Immunohistochemical detection of Glut3 in human tumors and normal tissues. Anticancer Res 1997; 17:2747–2750.
Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer 1997; 80:1046–1051.
Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, Molthoff CF. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 2002; 20:379–387.
Aoki D, Kawakami H, Nozawa S, Udagawa Y, Iizuka R, Hirano H. Differences in lectin binding patterns of normal human endometrium between proliferative and secretory phases. Histochemistry 1989; 92:177–184.
Mueckler M, Caruso C, Baldwin SA, et al. Sequence and structure of a human glucose transporter. Science 1985; 229:941–945.
White MK, Bramwell ME, Harris H. Kinetic parameters of hexose transport in hybrids between malignant and nonmalignant cells. J Cell Sci 1983; 62:49–80.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 1993; 34:1–6.
Kallinowski F, Schlenger KH, Runkel S, Kloes M, Stohrer M, Okunieff P, Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res 1989; 49:3759–3764.
Heber D, Byerley LO, Tchekmedyian NS. Hormonal and metabolic abnormalities in the malnourished cancer patient: effects on host-tumor interaction. JPEN J Parenter Enteral Nutr 1992; 16 (6 Suppl):60S–64S.
Musholt TJ, Musholt PB, Dehdashti F, Moley JF. Evaluation of fluorodeoxyglucose-positron emission tomographic scanning and its association with glucose transporter expression in medullary thyroid carcinoma and pheochromocytoma: a clinical and molecular study. Surgery 1997; 122:1049–1060.
Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 2003; 97:1015–1024.
Ito T, Noguchi Y, Satoh S, Hayashi H, Inayama Y, Kitamura H. Expression of facilitative glucose transporter isoforms in lung carcinomas: its relation to histologic type, differentiation grade, and tumor stage. Mod Pathol 1998; 11:437–443.
Reisser C, Eichhorn K, Herold-Mende C, Born AI, Bannasch P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int J Cancer 1999; 80:194–198.
Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res 1996; 2:1151–1154.
Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med 2001; 42:1551–1555.
Grobholz R, Hacker HJ, Thorens B, Bannasch P. Reduction in the expression of glucose transporter protein GLUT 2 in preneoplastic and neoplastic hepatic lesions and reexpression of GLUT 1 in late stages of hepatocarcinogenesis. Cancer Res 1993; 53:4204–4211.
Tai PK, Liao JF, Hossler PA, Castor CW, Carter-Su C. Regulation of glucose transporters by connective tissue activating peptide-III isoforms. J Biol Chem 1992; 267:19579–19586.
Wagstaff P, Kang HY, Mylott D, Robbins PJ, White MK. Characterization of the avian GLUT1 glucose transporter: differential regulation of GLUT1 and GLUT3 in chicken embryo fibroblasts. Mol Biol Cell 1995; 6:1575–1589.
Minn H, Lapela M, Klemi PJ, Grenman R, Leskinen S, Lindholm P, Bergman J, Eronen E, Haaparanta M, Joensuu H. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med 1997; 38:1907–1911.
Rege S, Safa AA, Chaiken L, Hoh C, Juillard G, Withers HR. Positron emission tomography: an independent indicator of radiocurability in head and neck carcinomas. Am J Clin Oncol 2000; 23:164–169.
Baer S, Casaubon L, Schwartz MR, Marcogliese A, Younes M. Glut3 expression in biopsy specimens of laryngeal carcinoma is associated with poor survival. Laryngoscope 2002; 112:393–396.
Acknowledgement
We thank Dr. Noboru Oriuchi and Dr. Tetsuya Higuchi of the Department of Nuclear Medicine, and Mrs.Mayumi Tomaru of the Department of Oral Surgery, Gunma University School of Medicine, for technical suggestions. We would also like to thank Professor Kuniaki Takata, Department of Anatomy Gunma University School of Medicine, and Professor Nobuaki Nakajima, Second Department of Pathology, Gunma University School of Medicine, for helpful suggestions with respect to pathology. This work was supported by grants from the Ministry of Education, Science and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, M., Zhang, H., Nakasone, Y. et al. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging 31, 5–12 (2004). https://doi.org/10.1007/s00259-003-1316-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1316-9