Abstract
Background
To investigate the differential expression of PD-1 and PD-L1 in salivary gland tumors (SGTs, malignant and benign subtypes) and determine their association with the clinicopathological characterization of the patients.
Methods
The immunohistochemistry was used to examine PD-1 and PD-L1 expression in specimens from 83 patients with primary SGTs including salivary ductal carcinoma (SDC), adenoid cystic carcinoma (AdCC), acinic cell carcinoma (ACC), mucoepidermoid carcinoma (MEC), warthin’s tumors (WT), poleomorphic adenoma (PA) and other subtypes.
Results
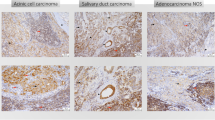
The expression of PD-1 in peripheral and central immune cells (ICs) of MEC, and peripheral ICs of ACC was significantly higher than those with AdCC (P = 0.02, P = 0.02, P = 0.03, respectively). Interestingly, the expression of PD-1 was also observed in peripheral and central malignant tumor cells (TCs), particularly in SDC and ACC. Despite no significant difference in PD-L1 expression of TCs among malignant subtypes, the peripheral and central ICs of ACC and MEC were revealed to express PDL-1 significantly more than those with AdCC (P < 0.05). WTs were rich in PD-1/PD-L1 expressing ICs. However, the tumor microenvironment of PA generally had low levels of PD-1/PD-L1 expression. In general, the expression of PD-1 in peripheral and central TCs was found to be significantly higher in malignant tumors than in benign ones (P = 0.002 and P = 0.003, respectively).
Conclusion
The simultaneous presentation of PD-1 and PD-L1 in TCs and ICs of SGTs, their significant association with disease severity as well as the positive correlation between these immune checkpoints may suggest the therapeutic potential of anti-PD-1 and anti-PDL-1 combinational immunotherapy for SGTs.





Similar content being viewed by others
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Code Availability
Antibody anti-PD-1 monoclonal antibody (CAT. NO. IHC001-100; GenomeMe Richmond, Canada); anti-PDL-1 monoclonal antibody (CAT. NO. IHC441-7; GenomeMe Richmond, Canada). Software IBM SPSS statistical software (version 21, SPSS Inc, USA), IBM SPSS Statistics (RRID: SCR_016479); Graph Pad Prism 6 software package (Inc; San Diego CA, USA, 2003), (RRID: SCR_002798).
References
Żurek M, Rzepakowska A, Jasak K, Niemczyk K. The epidemiology of salivary glands pathologies in adult population over 10 years in poland-cohort study. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph19010179.
Carlson ER, Schlieve T. Salivary gland malignancies. Oral Maxillofac Surg Clin North Am. 2019;31:125–44. https://doi.org/10.1016/j.coms.2018.08.007.
Bradley PJ. Classification of salivary gland neoplasms. Adv Otorhinolaryngol. 2016;78:1–8. https://doi.org/10.1159/000442119.
Sowa P, Goroszkiewicz K, Szydelko J, Chechlinska J, Pluta K, Domka W, et al. A review of selected factors of salivary gland tumour formation and malignant transformation. Biomed Res Int. 2018;2018:2897827. https://doi.org/10.1155/2018/2897827.
Mantravadi AV, Moore MG, Rassekh CH. AHNS series: do you know your guidelines? Diagnosis and management of salivary gland tumors. Head Neck. 2019;41:269–80. https://doi.org/10.1002/hed.25499.
Yu G, Peng X. Conservative and functional surgery in the treatment of salivary gland tumours. Int J Oral Sci. 2019;11:22. https://doi.org/10.1038/s41368-019-0059-9.
Amini A, Waxweiler TV, Brower JV, Jones BL, McDermott JD, Raben D, et al. Association of adjuvant chemoradiotherapy vs radiotherapy alone with survival in patients with resected major salivary gland carcinoma: data from the national cancer data base. JAMA Otolaryngol Head Neck Surg. 2016;142:1100–10. https://doi.org/10.1001/jamaoto.2016.2168.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68. https://doi.org/10.1038/s41577-020-0306-5.
Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12030738.
Ross JS, Gay LM, Wang K, Vergilio JA, Suh J, Ramkissoon S, et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann Oncol. 2017;28:2539–46. https://doi.org/10.1093/annonc/mdx399.
Kato S, Elkin SK, Schwaederle M, Tomson BN, Helsten T, Carter JL, et al. Genomic landscape of salivary gland tumors. Oncotarget. 2015;6:25631–45. https://doi.org/10.18632/oncotarget.4554.
Vital D, Ikenberg K, Moch H, Rössle M, Huber GF. The expression of PD-L1 in salivary gland carcinomas. Sci Rep. 2019;9:12724. https://doi.org/10.1038/s41598-019-49215-9.
Sato F, Ono T, Kawahara A, Matsuo K, Kondo R, Sato K, et al. Prognostic value of tumor proportion score in salivary gland carcinoma. Laryngoscope. 2021;131:E1481–8. https://doi.org/10.1002/lary.29120.
Kuchar M, Strizova Z, Capkova L, Komarc M, Skrivan J, Bartunkova J, et al. The periphery of salivary gland carcinoma tumors reveals a PD-L1/PD-1 biomarker niche for the evaluation of disease severity and tumor-immune system interplay. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9020097.
Porcheri C, Meisel CT, Mitsiadis TA. Molecular and cellular modelling of salivary gland tumors open new landscapes in diagnosis and treatment. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12113107.
Witte HM, Gebauer N, Lappöhn D, Umathum VG, Riecke A, Arndt A, et al. Prognostic impact of PD-L1 expression in malignant salivary gland tumors as assessed by established scoring criteria: tumor proportion score (TPS), combined positivity score (CPS), and immune cell (IC) infiltrate. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12040873.
Fang Q, Wu Y, Du W, Zhang X, Chen D. Incidence and prognostic significance of PD-L1 expression in high-grade salivary gland carcinoma. Front Oncol. 2021;11:701181. https://doi.org/10.3389/fonc.2021.701181.
Kuzenko YV, Romanuk AM, Dyachenko OO, Hudymenko O. Pathogenesis of warthin’s tumors. Interv Med Appl Sci. 2016;8:41–8. https://doi.org/10.1556/1646.8.2016.2.2.
Haghshenas MR, Khademi B, Faghih Z, Ghaderi A, Erfani N. Immune regulatory cells and IL17-producing lymphocytes in patients with benign and malignant salivary gland tumors. Immunol Lett. 2015;164:109–16. https://doi.org/10.1016/j.imlet.2015.02.008.
Haghshenas MR, Khademi B, Ashraf MJ, Ghaderi A, Erfani N. Helper and cytotoxic T-cell subsets (Th1, Th2, Tc1, and Tc2) in benign and malignant salivary gland tumors. Oral Dis. 2016;22:566–72. https://doi.org/10.1111/odi.12496.
Haghshenas MR, Ghaderi H, Daneste H, Ghaderi A. Immunological and biological dissection of normal and tumoral salivary glands. Int Rev Immunol. 2021. https://doi.org/10.1080/08830185.2021.1958806.
Wang X, Yang X, Zhang C, Wang Y, Cheng T, Duan L, et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc Natl Acad Sci USA. 2020;117:6640–50. https://doi.org/10.1073/pnas.1921445117.
Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66:1920–33. https://doi.org/10.1002/hep.29360.
Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162:1242–56. https://doi.org/10.1016/j.cell.2015.08.052.
Sakai M, Fukumoto M, Ikai K, Ono Minagi H, Inagaki S, Kogo M, et al. Role of the mTOR signalling pathway in salivary gland development. Febs j. 2019;286:3701–17. https://doi.org/10.1111/febs.14937.
Diegel CR, Cho KR, El-Naggar AK, Williams BO, Lindvall C. Mammalian target of rapamycin-dependent acinar cell neoplasia after inactivation of Apc and Pten in the mouse salivary gland: implications for human acinic cell carcinoma. Cancer Res. 2010;70:9143–52. https://doi.org/10.1158/0008-5472.Can-10-1758.
Sato T, Maeta T, Abe R, Yamada H, Ishida K, Yashima-Abo A, et al. Successful treatment with nivolumab in a patient with metastatic salivary duct carcinoma. Case Rep Oncol. 2021;14:343–6. https://doi.org/10.1159/000512060.
Chang H, Kim JS, Choi YJ, Cho JG, Woo JS, Kim A, et al. Overexpression of PD-L2 is associated with shorter relapse-free survival in patients with malignant salivary gland tumors. Onco Targets Ther. 2017;10:2983–92. https://doi.org/10.2147/ott.S134589.
Mosconi C, de Arruda JAA, de Farias ACR, Oliveira GAQ, de Paula HM, Fonseca FP, et al. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol. 2019;88:95–101. https://doi.org/10.1016/j.oraloncology.2018.11.028.
Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. https://doi.org/10.1016/j.canlet.2019.10.013.
Tang YL, Fan YL, Jiang J, Li KD, Zheng M, Chen W, et al. C-kit induces epithelial-mesenchymal transition and contributes to salivary adenoid cystic cancer progression. Oncotarget. 2014;5:1491–501. https://doi.org/10.18632/oncotarget.1606.
Enescu AS, Mărgăritescu CL, Crăiţoiu MM, Enescu A, Crăiţoiu Ş. The involvement of growth differentiation factor 5 (GDF5) and aggrecan in the epithelial-mesenchymal transition of salivary gland pleomorphic adenoma. Rom J Morphol Embryol. 2013;54:969–76.
Hellquist H, Paiva-Correia A, Vander Poorten V, Quer M, Hernandez-Prera JC, Andreasen S, et al. Analysis of the clinical relevance of histological classification of benign epithelial salivary gland tumours. Adv Ther. 2019;36:1950–74. https://doi.org/10.1007/s12325-019-01007-3.
Chin KW, Billings KR, Ishiyama A, Wang MB, Wackym PA. Characterization of lymphocyte subpopulations in warthin’s tumor. Laryngoscope. 1995;105:928–33. https://doi.org/10.1288/00005537-199509000-00011.
O’Neill ID. New insights into the nature of warthin’s tumour. J Oral Pathol Med. 2009;38:145–9. https://doi.org/10.1111/j.1600-0714.2008.00676.x.
Triantafyllou A, Thompson LD, Devaney KO, Bell D, Hunt JL, Rinaldo A, et al. Functional histology of salivary gland pleomorphic adenoma: an appraisal. Head Neck Pathol. 2015;9:387–404. https://doi.org/10.1007/s12105-014-0581-1.
Brodetskyi IS, Dyadyk OO, Myroshnychenko MS, Zaritska VI. Morphological characteristics of pleomorphic adenomas of salivary glands (analysis of the surgical material). Wiad Lek. 2020;73:2339–44.
Hiss S, Eckstein M, Segschneider P, Mantsopoulos K, Iro H, Hartmann A, et al. Tumour-infiltrating lymphocytes (TILs) and PD-L1 expression correlate with lymph node metastasis, high-grade transformation and shorter metastasis-free survival in patients with acinic cell carcinoma (AciCC) of the salivary glands. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13050965.
Harada K, Ferdous T, Ueyama Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer. 2018;18:156. https://doi.org/10.1186/s12885-018-4069-3.
Strizova Z, Taborska P, Stakheev D, Partlová S, Havlova K, Vesely S, et al. NK and T cells with a cytotoxic/migratory phenotype accumulate in peritumoral tissue of patients with clear cell renal carcinoma. Urol Oncol. 2019;37:503–9. https://doi.org/10.1016/j.urolonc.2019.03.014.
Strizova Z, Snajdauf M, Stakheev D, Taborska P, Vachtenheim J Jr, Biskup J, et al. The paratumoral immune cell signature reveals the potential for the implementation of immunotherapy in esophageal carcinoma patients. J Cancer Res Clin Oncol. 2020;146:1979–92. https://doi.org/10.1007/s00432-020-03258-y.
Rodriguez CP, Wu QV, Voutsinas J, Fromm JR, Jiang X, Pillarisetty VG, et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. 2020;26:837–45. https://doi.org/10.1158/1078-0432.Ccr-19-2214.
Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol. 2018;41:1083–8. https://doi.org/10.1097/coc.0000000000000429.
Zhang J, Fang W, Qin T, Yang Y, Hong S, Liang W, et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015;32:86. https://doi.org/10.1007/s12032-015-0501-6.
Yin H, Tang Y, Guo Y, Wen S. Immune microenvironment of thyroid cancer. J Cancer. 2020;11:4884–96. https://doi.org/10.7150/jca.44506.
Mahmood U, Bang A, Chen YH, Mak RH, Lorch JH, Hanna GJ, et al. A randomized phase 2 study of pembrolizumab with or without radiation in patients with recurrent or metastatic adenoid cystic carcinoma. Int J Radiat Oncol Biol Phys. 2021;109:134–44. https://doi.org/10.1016/j.ijrobp.2020.08.018.
Giraldo NA, Becht E, Pagès F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21:3031–40. https://doi.org/10.1158/1078-0432.Ccr-14-2926.
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34. https://doi.org/10.1038/nrclinonc.2017.101.
Acknowledgements
This project was conducted as the MD thesis of Sajjad Gerdabi, and was financially supported by grants from Shiraz University of Medical Sciences, Shiraz, Iran (Grant No: 22827), as well as Shiraz Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran (ICR-100-500).
Funding
This study was supported by a Master’s thesis grant from Shiraz University of Medical Sciences (Grant number: 22827) as well as from Shiraz Institute for Cancer Research (Grant number: ICR-100–500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There was no conflict of interest among the authors References.
Ethical approval
The study was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.371).
Consent to participate
All patients provided informed written consent.
Consent for publication
The Authors informed consent to publication of the Work in any publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gerdabi, S., Asadian, F., Kiani, R. et al. Simultaneous Expression of PD-1 and PD-L1 in Peripheral and Central Immune Cells and Tumor Cells in the Benign and Malignant Salivary Gland Tumors Microenvironment. Head and Neck Pathol 17, 178–192 (2023). https://doi.org/10.1007/s12105-022-01486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01486-x