Abstract
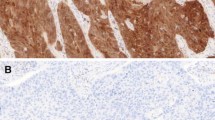
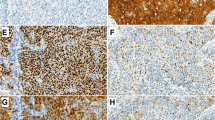
While P16 immunohistochemistry (IHC) is a well-established surrogate marker of Human Papillomavirus (HPV) in oropharyngeal squamous cell carcinoma (OSCC), Retinoblastoma 1 (RB1) loss may lead to p16 overexpression in the absence of HPV. We determined the proportion of p16-positive/HPV-negative OSCC with RB1 loss and other alterations in RB1/p16 pathway, and tested RB1 IHC as a prognostic biomarker for OSCC, along with the 8th edition of AJCC staging manual. P16 and RB1 IHC and HPV DNA in situ hybridization (ISH) were performed on 257 OSCC. High risk HPV RNA ISH, RB1 fluorescence in situ hybridization (FISH), and next generation sequencing (NGS) were done on p16-positive/HPV DNA ISH-negative OSCC. Disease free survival (DFS) was used as an endpoint. In the entire cohort and in p16-positive (n = 184) and p16-negative (n = 73) subgroups, AJCC 8th edition staging correlated with DFS (p < 0.01). RB1 IHC showed RB1 loss in p16-positive OSCC only (79/184, 43%). RB1 loss by IHC is associated with a better DFS, without providing additional prognostic information for patients with p16-positive OSCC. HPV RNA ISH was positive in 12 of 14 HPV DNA ISH-negative cases. RB1 IHC showed loss in 10 of 15 HPV DNA ISH-negative cases and in 1 of 2 HPV RNA ISH-negative cases. Overall, only one case of p16-positive/HPV RNA ISH-negative OSCC showed RB1 loss by IHC (1/184, 0.5%). Of the 10 p16-positive and HPV DNA ISH-negative cases with RB1 loss by IHC, 2 had RB1 hemizygous deletion and 3 showed Chromosome 13 monosomy by FISH. No RB1 mutations were detected by NGS. Other molecular alterations in p16-positive/HPV DNA ISH-negative cases included TP53 and TERT mutations and DDX3X loss. HPV-independent RB1 inactivation rarely results in false positive p16 IHC. RB1 inactivation by high risk HPV E7 oncoprotein may co-exist with RB1 deletion. RB1 loss is a favorable prognosticator and occurs exclusively in p16-positive OSCC. The 8th edition of the AJCC staging manual satisfactorily predicts DFS of OSCC patients.





Similar content being viewed by others
References
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. https://doi.org/10.1056/NEJMoa0912217.
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. https://doi.org/10.1093/jnci/djn011.
Amin MBE, S.B. Greene, F. et al. AJCC Cancer Staging Manual 8th ed. New York, NY: Springer. 2017.
Lewis JS Jr, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of american pathologists. Arch Pathol Lab Med. 2018;142(5):559–97. https://doi.org/10.5858/arpa.2017-0286-CP.
Holzinger D, Flechtenmacher C, Henfling N, Kaden I, Grabe N, Lahrmann B, et al. Identification of oropharyngeal squamous cell carcinomas with active HPV16 involvement by immunohistochemical analysis of the retinoblastoma protein pathway. Int J Cancer. 2013;133(6):1389–99. https://doi.org/10.1002/ijc.28142.
Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Can Res. 1996;56(20):4620–4.
Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264(1):42–55. https://doi.org/10.1006/excr.2000.5149.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36.
Lechner M, Chakravarthy AR, Walter V, Masterson L, Feber A, Jay A, et al. Frequent HPV-independent p16/INK4A overexpression in head and neck cancer. Oral Oncol. 2018;83:32–7. https://doi.org/10.1016/j.oraloncology.2018.06.006.
Li Y, Nichols MA, Shay JW, Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Can Res. 1994;54(23):6078–82.
Plath M, Broglie MA, Forbs D, Stoeckli SJ, Jochum W. Prognostic significance of cell cycle-associated proteins p16, pRB, cyclin D1 and p53 in resected oropharyngeal carcinoma. J Otolaryngol Head Neck Surg. 2018;47(1):53. https://doi.org/10.1186/s40463-018-0298-3.
Svajdler M, Mezencev R, Ondic O, Saskova B, Mukensnabl P, Michal M. P16 is a useful supplemental diagnostic marker of pulmonary small cell carcinoma in small biopsies and cytology specimens. Ann Diagn Pathol. 2018;33:23–9. https://doi.org/10.1016/j.anndiagpath.2017.11.008.
Murao K, Kubo Y, Ohtani N, Hara E, Arase S. Epigenetic abnormalities in cutaneous squamous cell carcinomas: frequent inactivation of the RB1/p16 and p53 pathways. Br J Dermatol. 2006;155(5):999–1005. https://doi.org/10.1111/j.1365-2133.2006.07487.x.
Ryu CH, Ryu J, Cho KH, Moon SH, Yun T, Lee SH, et al. Human papillomavirus-related cell cycle markers can predict survival outcomes following a transoral lateral oropharyngectomy for tonsillar squamous cell carcinoma. J Surg Oncol. 2014;110(4):393–9. https://doi.org/10.1002/jso.23672.
Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, Conradt C, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Can Res. 1998;58(1):5–13.
Beck TN, Smith CH, Flieder DB, Galloway TJ, Ridge JA, Golemis EA, et al. Head and neck squamous cell carcinoma: Ambiguous human papillomavirus status, elevated p16, and deleted retinoblastoma 1. Head Neck. 2017;39(3):E34–9. https://doi.org/10.1002/hed.24604.
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39(6):744–52. https://doi.org/10.1097/PAS.0000000000000410.
Chenevert J, Chiosea S. Incidence of human papillomavirus in oropharyngeal squamous cell carcinomas: now and 50 years ago. Hum Pathol. 2012;43(1):17–22. https://doi.org/10.1016/j.humpath.2011.03.009.
Rooper LM, Gandhi M, Bishop JA, Westra WH. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–6. https://doi.org/10.1016/j.oraloncology.2016.02.008.
Nikiforova MN, Wald AI, Melan MA, Roy S, Zhong S, Hamilton RL, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3):379–87. https://doi.org/10.1093/neuonc/nov289.
Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–37. doi:https://doi.org/10.3322/caac.21389.
Keung ES, Souers RJ, Bridge JA, Faquin WC, Graham RP, Hameed MR, et al. Comparative performance of high-risk human papillomavirus RNA and DNA in situ hybridization on college of American pathologists proficiency tests. Arch Pathol Lab Med. 2020;144(3):344–9. https://doi.org/10.5858/arpa.2019-0093-CP.
Boscolo-Rizzo P, Da Mosto MC, Rampazzo E, Giunco S, Del Mistro A, Menegaldo A, et al. Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications. Cancer Metastasis Rev. 2016;35(3):457–74. https://doi.org/10.1007/s10555-016-9633-1.
Resteghini C, Perrone F, Miceli R, Bergamini C, Alfieri S, Orlandi E, et al. Prognostic role of PIK3CA and TP53 in human papillomavirus-negative oropharyngeal cancers. Tumori. 2018. https://doi.org/10.1177/0300891618765558.
Wichmann G, Rosolowski M, Krohn K, Kreuz M, Boehm A, Reiche A, et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int J Cancer. 2015;137(12):2846–57. https://doi.org/10.1002/ijc.29649.
Lee CH, Lin SH, Yang SF, Yang SM, Chen MK, Lee H, et al. Low/negative expression of DDX3 might predict poor prognosis in non-smoker patients with oral cancer. Oral Dis. 2014;20(1):76–83. https://doi.org/10.1111/odi.12076.
Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–41. https://doi.org/10.1158/1078-0432.Ccr-13-3310.
Funding
No funding obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
All tests performed in this retrospective study involving human participants were in accordance with the ethical standards of the institutional review board (IRB991206), which did not require informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berdugo, J., Rooper, L.M. & Chiosea, S.I. RB1, p16, and Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma. Head and Neck Pathol 15, 1109–1118 (2021). https://doi.org/10.1007/s12105-021-01317-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01317-5