Abstract
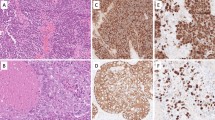
Epithelial-myoepithelial carcinoma (EMC) can be a challenging diagnosis due to a lack of obvious invasion and bland cytology. We report an unusual case of a low-grade EMC with prominent fibrous stroma, an extensive solid-oncocytic differentiation and limited areas of morphological clearly identifiable characteristic biphasic (tubular) differentiation, clear cells and PAS-positive secretions/calcifications. Both areas were investigated by next generation sequencing (Oncomine comprehensive assay) and revealed a typical concordant HRAS p.Q61R mutation. An additional heterogeneous ARID1A (p.E672*) terminating mutation with loss of heterozygosity, which could be visualized predominantly in the solid-oncocytic differentiation by immunohistochemical loss of ARID1A protein expression, was found. This is the first case of an EMC of the salivary gland to be described with two separate tumor clones involving concordant HRAS and heterogeneous ARID1A mutations. The latter seem to be a “second hit” and was predominantly found in the solid-oncocytic differentiation, suggesting a potential morpho-molecular association.


Similar content being viewed by others
References
Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31(1):44–57. https://doi.org/10.1097/01.pas.0000213314.74423.d8.
Yang GC, Soslow RA. Epithelial-myoepithelial carcinoma of the parotid. A case of ductal-predominant presentation with cytologic, histologic and ultrastructural correlations. Acta Cytol. 1999;43(6):1113–8. https://doi.org/10.1159/000331363.
El Hallani S, Udager AM, Bell D, Fonseca I, Thompson LDR, Assaad A, et al. Epithelial-myoepithelial carcinoma: frequent morphologic and molecular evidence of preexisting pleomorphic adenoma, common HRAS mutations in PLAG1-intact and HMGA2-intact cases, and occasional TP53, FBXW7, and SMARCB1 alterations in high-grade cases. Am J Surg Pathol. 2018;42(1):18–27. https://doi.org/10.1097/PAS.0000000000000933.
Seethala RR, Richmond JA, Hoschar AP, Barnes EL. New variants of epithelial-myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med. 2009;133(6):950–9. https://doi.org/10.1043/1543-2165-133.6.950.
Seethala RR. Oncocytic and apocrine epithelial myoepithelial carcinoma: novel variants of a challenging tumor. Head Neck Pathol. 2013;7(Suppl 1):S77–84. https://doi.org/10.1007/s12105-013-0461-0.
Chiosea SI, Miller M, Seethala RR. HRAS mutations in epithelial-myoepithelial carcinoma. Head Neck Pathol. 2014;8(2):146–50. https://doi.org/10.1007/s12105-013-0506-4.
Urano M, Nakaguro M, Yamamoto Y, Hirai H, Tanigawa M, Saigusa N, et al. Diagnostic significance of HRAS mutations in epithelial-myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am J Surg Pathol. 2019. https://doi.org/10.1097/PAS.0000000000001258.
Geyer FC, Li A, Papanastasiou AD, Smith A, Selenica P, Burke KA, et al. Recurrent hotspot mutations in HRAS Q61 and PI3 K-AKT pathway genes as drivers of breast adenomyoepitheliomas. Nat Commun. 2018;9(1):1816. https://doi.org/10.1038/s41467-018-04128-5.
Pareja F, Geyer FC, Brown DN, Sebastiao APM, Gularte-Merida R, Li A, et al. Assessment of HMGA2 and PLAG1 rearrangements in breast adenomyoepitheliomas. NPJ Breast Cancer. 2019;5:6. https://doi.org/10.1038/s41523-018-0101-7.
Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 2013;3(1):35–43. https://doi.org/10.1158/2159-8290.CD-12-0361.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43. https://doi.org/10.1056/NEJMoa1008433.
Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–56. https://doi.org/10.1002/gcc.22195.
Sebastiao APM, Pareja F, Kumar R, Brown DN, Silveira C, da Silva EM, et al. Genomic analysis of recurrences and high-grade forms of polymorphous adenocarcinomas. Histopathology. 2019. https://doi.org/10.1111/his.13854.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–13. https://doi.org/10.1038/nm.4333.
Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–31. https://doi.org/10.1126/science.1196333.
Williamson CT, Miller R, Pemberton HN, Jones SE, Campbell J, Konde A, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837. https://doi.org/10.1038/ncomms13837.
Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5(7):752–67. https://doi.org/10.1158/2159-8290.CD-14-0849.
Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P. Loss of ARID1A expression sensitizes cancer cells to PI3 K- and AKT-inhibition. Oncotarget. 2014;5(14):5295–303. https://doi.org/10.18632/oncotarget.2092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing financial interests exist.
Ethical approval
The patient provided a written informed consent in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rupp, N.J., Brada, M., Skálová, A. et al. New Insights into Tumor Heterogeneity: A Case of Solid-Oncocytic Epithelial-Myoepithelial Carcinoma of the Parotid Gland Harboring a HRAS and Heterogeneous Terminating ARID1A Mutation. Head and Neck Pathol 14, 554–558 (2020). https://doi.org/10.1007/s12105-019-01055-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01055-9