Abstract
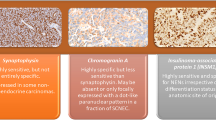
Endolymphatic sac tumors (ELSTs) are rare, slowly growing temporal bone neoplasms which show a high association with von Hippel-Lindau (VHL) syndrome. The immunohistochemistry evaluation of these papillary-cystic neoplasms frequently raises the differential diagnosis with renal cell carcinoma, among other metastatic neoplasms, whether in VHL patients or not. A cohort of 26 patients with ELSTs were evaluated for histologic features, immunohistochemistry findings, and association with VHL. Standard immunohistochemistry evaluation was performed. Sixteen females and 10 males ranging in age from 10 to 69 years (mean 44; VHL mean: 32) at initial presentation, comprised the cohort of patients. Most (86%) experienced hearing changes or inner ear symptoms (vertigo, dizziness), with an average duration of symptoms for 39 months (range 2–240 months). The tumors were an average of 2.9 cm (range 0.4–8 cm), with 14 left, 11 right sided and one bilateral tumor. Nine patients had documented VHL, with 3 patients having a concurrent or subsequent clear cell renal cell carcinoma. Patients were followed an average of 6.2 years (available in 24 patients): 19 alive without disease, 7.5 years; 2 dead without disease, 1.2 years; and 3 alive with disease, 3.1 years. The neoplastic cells show the following immunohistochemistry findings: AE1/AE3, EMA, CK7, CAIX, GLUT1, VEGF: 100% of cases tested were positive; pax-8: 85% of cases positive; CD10 and RCC: 0% of cases reactive. Based on this cohort of 26 patients with ELST, 9 of whom had VHL, the strong pax-8 and CAIX should be used in conjunction with negative CD10 and RCC to help exclude a metastatic renal cell carcinoma. As CAIX is an enzyme overexpressed in hypoxia and hypoxia inducible factor is what VHL protein regulates, this is an expected, although previously unreported finding. Whether part of VHL or not, VHL mutations may be a somatic rather than germline finding in the tumors, a possible further explanation for the CAIX reaction.







Similar content being viewed by others
References
Heffner DK. Low-grade adenocarcinoma of probable endolymphatic sac origin A clinicopathologic study of 20 cases. Cancer. 1989;64:2292–302.
Skalova A, Sima R, Bohus P, Curik R, Lukas J, Michal M. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): report of two cases with analysis of the VHL gene. Pathol Res Pract. 2008;204:599–606.
Manski TJ, Heffner DK, Glenn GM, et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997;277:1461–6.
Delisle MB, Uro E, Rouquette I, Yardeni E, Rumeau JL. Papillary neoplasm of the endolymphatic sac in a patient with von Hippel-Lindau disease. J Clin Pathol. 1994;47:959–61.
Bausch B, Wellner U, Peyre M, et al. Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the International Endolymphatic Sac Tumor Registry. Head Neck. 2016;38(Suppl 1):E673–9.
Michaels L. Origin of endolymphatic sac tumor. Head Neck Pathol. 2007;1:104–11.
Hassard AD, Boudreau SF, Cron CC. Adenoma of the endolymphatic sac. J Otolaryngol. 1984;13:213–6.
Mukherji SK, Albernaz VS, Lo WW, et al. Papillary endolymphatic sac tumors: CT, MR imaging, and angiographic findings in 20 patients. Radiology. 1997;202:801–8.
Folker RJ, Meyerhoff WL, Rushing EJ. Aggressive papillary adenoma of the cerebellopontine angle: case report of an endolymphatic sac tumor. Am J Otolaryngol. 1997;18:135–9.
Gaffey MJ, Mills SE, Fechner RE, Intemann SR, Wick MR. Aggressive papillary middle-ear tumor. A clinicopathologic entity distinct from middle-ear adenoma. Am J Surg Pathol. 1988;12:790–7.
Meyer JR, Gebarski SS, Blaivas M. Cerebellopontine angle invasive papillary cystadenoma of endolymphatic sac origin with temporal bone involvement. AJNR Am J Neuroradiol. 1993;14:1319–21. discussion 1322 – 1313.
Lavoie M, Morency RM. Low-grade papillary adenomatous tumors of the temporal bone: report of two cases and review of the literature. Mod Pathol. 1995;8:603–8.
Bisceglia M, D’Angelo VA, Wenig BM. Endolymphatic sac papillary tumor (Heffner tumor). Adv Anat Pathol. 2006;13:131–8.
Yu SJ, Chen YD, Gao F, Qiu XG, Chang H. Endolymphatic sac papillary tumor: a case report. Chin Med J (Engl). 2011;124:3828–9.
Malhotra S, Rao RV, Valiathan M, Mathew M, Nayak DR, Raja A. Low-grade adenocarcinoma of endolymphatic sac origin. Am J Otolaryngol. 2006;27:362–5.
Monedero Martinez-Pardo E, Navia Alvarez P. Endolymphatic sac carcinoma: a case report. Radiologia. 2011;53:483–4.
Mukherji SK, Castillo M. Adenocarcinoma of the endolymphatic sac: imaging features and preoperative embolization. Neuroradiology. 1996;38:179–80.
Wang HQ, Jie L, Shi HY. Clinicopathological features of low-grade malignant endolymphatic sac tumors. Pathol Res Pract. 2018;214:431–5.
Batsakis JG, El-Naggar AK. Papillary neoplasms (Heffner’s tumors) of the endolymphatic sac. Ann Otol Rhinol Laryngol. 1993;102:648–51.
Glasker S, Lonser RR, Tran MG, et al. Effects of VHL deficiency on endolymphatic duct and sac. Cancer Res. 2005;65:10847–53.
Andreasen S, Therkildsen MH, Grauslund M, Friis-Hansen L, Wessel I, Homoe P. Activation of the interleukin-6/Janus kinase/STAT3 pathway in pleomorphic adenoma of the parotid gland. Apmis. 2015;123:706–15.
Bellairs JA, Gluth MB. A histopathological connection between a fatal endolymphatic sac tumour and von Hippel-Lindau disease from 1960. J Laryngol Otol. 2018;132:75–8.
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–23.
Kamura T, Koepp DM, Conrad MN, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–61.
Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5.
Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8.
Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72.
Kaelin WG Jr. Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. Cancer. 2009;115:2262–72.
Wick CC, Manzoor NF, Semaan MT, Megerian CA. Endolymphatic sac tumors. Otolaryngol Clin North Am. 2015;48:317–30.
Timmer FC, Neeskens LJ, van den Hoogen FJ, et al. Endolymphatic sac tumors: clinical outcome and management in a series of 9 cases. Otol Neurotol. 2011;32:680–5.
Codreanu CM, Duet M, Hautefort C, et al. Endolymphatic sac tumors in von Hippel-Lindau disease: report of three cases. Otol Neurotol. 2010;31:660–4.
Rao Q, Zhou J, Wang JD, et al. Endolymphatic sac tumor with von Hippel-Lindau disease: report of a case with analysis of von Hippel-Lindau gene and review. Ann Diagn Pathol. 2010;14:361–4.
Kim HJ, Butman JA, Brewer C, et al. Tumors of the endolymphatic sac in patients with von Hippel-Lindau disease: implications for their natural history, diagnosis, and treatment. J Neurosurg. 2005;102:503–12.
Vortmeyer AO, Huang SC, Koch CA, et al. Somatic von Hippel-Lindau gene mutations detected in sporadic endolymphatic sac tumors. Cancer Res. 2000;60:5963–5.
Vortmeyer AO, Choo D, Pack S, Oldfield E, Zhuang Z. VHL gene inactivation in an endolymphatic sac tumor associated with von Hippel-Lindau disease. Neurology. 2000;55:460.
Vortmeyer AO, Choo D, Pack SD, Oldfield E, Zhuang Z. von Hippel-Lindau disease gene alterations associated with endolymphatic sac tumor. J Natl Cancer Inst. 1997;89:970–2.
Hamazaki S, Yoshida M, Yao M, et al. Mutation of von Hippel-Lindau tumor suppressor gene in a sporadic endolymphatic sac tumor. Hum Pathol. 2001;32:1272–6.
Kim HJ, Hagan M, Butman JA, et al. Surgical resection of endolymphatic sac tumors in von Hippel-Lindau disease: findings, results, and indications. Laryngoscope. 2013;123:477–83.
Bell D, Gidley P, Levine N, Fuller GN. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): sine qua non radiology-pathology and the University of Texas MD Anderson Cancer Center experience. Ann Diagn Pathol. 2011;15:117–23.
Jensen RL, Gillespie D, House P, Layfield L, Shelton C. Endolymphatic sac tumors in patients with and without von Hippel-Lindau disease: the role of genetic mutation, von Hippel-Lindau protein, and hypoxia inducible factor-1alpha expression. J Neurosurg. 2004;100:488–97.
Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol. 2014;4:400.
Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–83.
Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–26.
Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:293–9.
Ozcan A, Shen SS, Hamilton C, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–64.
Hu Y, Hartmann A, Stoehr C, et al. PAX8 is expressed in the majority of renal epithelial neoplasms: an immunohistochemical study of 223 cases using a mouse monoclonal antibody. J Clin Pathol. 2012;65:254–6.
Ordonez NG. Value of PAX8, PAX2, napsin A, carbonic anhydrase IX, and claudin-4 immunostaining in distinguishing pleural epithelioid mesothelioma from metastatic renal cell carcinoma. Mod Pathol. 2013;26:1132–43.
Mentrikoski MJ, Wendroth SM, Wick MR. Immunohistochemical distinction of renal cell carcinoma from other carcinomas with clear-cell histomorphology: utility of CD10 and CA-125 in addition to PAX-2, PAX-8, RCCma, and adipophilin. Appl Immunohistochem Mol Morphol. 2014;22:635–41.
Magers MJ, Udager AM, Chinnaiyan AM, et al. Comprehensive Immunophenotypic Characterization of Adult and Fetal Testes, the Excretory Duct System, and Testicular and Epididymal Appendages. Appl Immunohistochem Mol Morphol. 2016;24:e50–68.
Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–82.
Johnson Chacko L, Pechriggl EJ, Fritsch H, et al. Neurosensory differentiation and innervation patterning in the human fetal vestibular end organs between the gestational weeks 8–12. Front Neuroanat. 2016;10:111.
DeJonge RE, Liu XP, Deig CR, Heller S, Koehler KR, Hashino E. Modulation of Wnt signaling enhances inner ear organoid development in 3D culture. PLoS ONE. 2016;11:e0162508.
Horiguchi H, Sano T, Toi H, Kageji T, Hirokawa M, Nagahiro S. Endolymphatic sac tumor associated with a von Hippel-Lindau disease patient: an immunohistochemical study. Mod Pathol. 2001;14:727–32.
Megerian CA, Pilch BZ, Bhan AK, McKenna MJ. Differential expression of transthyretin in papillary tumors of the endolymphatic sac and choroid plexus. Laryngoscope. 1997;107:216–21.
Megerian CA, McKenna MJ, Nuss RC, et al. Endolymphatic sac tumors: histopathologic confirmation, clinical characterization, and implication in von Hippel-Lindau disease. Laryngoscope. 1995;105:801–8.
Du J, Wang J, Cui Y, et al. Clinicopathologic study of endolymphatic sac tumor (ELST) and differential diagnosis of papillary tumors located at the cerebellopontine angle. Neuropathology. 2015;35:410–20.
Zanoletti E, Girasoli L, Borsetto D, Opocher G, Mazzoni A, Martini A. Endolymphatic sac tumour in von Hippel-Lindau disease: management strategies. Acta Otorhinolaryngol Ital. 2017;37:423–9.
Bastier PL, de Mones E, Marro M, et al. Endolymphatic sac tumors: experience of three cases. Eur Arch Otorhinolaryngol. 2013;270:1551–7.
Acknowledgements
Presented at the 106th Annual Meeting of the United States and Canadian Academy of Pathology, Vancouver, British Columbia, Canada, March, 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest as it relates to this research project. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968), which did not require informed consent.
Rights and permissions
About this article
Cite this article
Thompson, L.D.R., Magliocca, K.R., Andreasen, S. et al. CAIX and pax-8 Commonly Immunoreactive in Endolymphatic Sac Tumors: A Clinicopathologic Study of 26 Cases with Differential Considerations for Metastatic Renal Cell Carcinoma in von Hippel-Lindau Patients. Head and Neck Pathol 13, 355–363 (2019). https://doi.org/10.1007/s12105-018-0973-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0973-8