Abstract
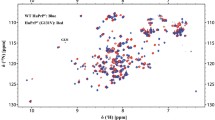
Alpha-synuclein (α-syn) is a small presynaptic protein that is believed to play an important role in the pathogenesis of Parkinson's disease (PD). It localizes to presynaptic terminals where it partitions between a cytosolic soluble and a lipid-bound state. Recent evidence suggests that α-syn can also associate with mitochondrial membranes where it interacts with a unique anionic phospholipid cardiolipin (CL). Here, we examine the conformation of the flexible fragments of a monomeric α-syn bound to lipid vesicles composed of anionic 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) lipids, of tetraoleoyl CL (TOCL) and DOPC, and of fibrils. The dynamic properties of α-syn associated with DOPA:DOPC vesicles were the most favorable for conducting three-dimensional NMR experiments, and the 13C, 15N and amide 1H chemical shifts of the flexible and disordered C-terminus of α-syn could be assigned using three-dimensional through-bond magic angle spinning NMR spectroscopy. Although the C-terminus is more dynamically constrained in fibrils and in α-syn bound to TOCL:DOPC vesicles, a direct comparison of carbon chemical shifts detected using through bond two-dimensional spectroscopy indicates that the C-terminus is flexible and unstructured in all the three samples.



Similar content being viewed by others
Availability of data and materials
The data leading to the assignment could be obtained from the authors upon request.
References
Abeliovich A, Schmitz Y, Farinas I et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252
Ambasudhan R, Ryan SD, Dolatabadi N et al (2013) Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell 155:1351
Bodles AM, Guthrie DJS, Greer B, Brent Irvine G (2001) Identification of the region of non-Aβ component (NAC) of Alzheimer’s disease amyloid responsible for its aggregation and toxicity. J Neurochem 78:384–395
Bodner CR, Dobson CM, Bax A (2009) Multiple tight phospholipid-binding modes of alpha-synuclein revealed by solution NMR spectroscopy. J Mol Biol 390:775–790
Burre J, Sharma M, Tsetsenis T et al (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667
Chandra S, Gallardo G, Fernandez-Chacon R et al (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396
Chu CT, Ji J, Dagda RK et al (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15:1197–1205
Cole NB, Dieuliis D, Leo P et al (2008) Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res 314:2076–2089
Comellas G, Lemkau LR, Nieuwkoop AJ et al (2011) Structured regions of α-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J Mol Biol 411:881–895
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:880–909
Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449
Delaglio F, Grzesiek S, Vuister GW et al (1995) Nmrpipe—a multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6:277–293
Eliezer D, Kutluay E, Bussell R, Browne G (2001) Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol 307:1061–1073
Falk AS, Siemer AB (2016) Dynamic domains of amyloid fibrils can be site-specifically assigned with proton detected 3D NMR spectroscopy. J Biomol NMR 66:159–162
Flower TR, Chesnokova LS, Froelich CA et al (2005) Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J Mol Biol 351:1081–1100
Fusco G, De Simone A, Gopinath T et al (2014) Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat Commun 5:3827
Gath J, Habenstein B, Bousset L et al (2012) Solid-state NMR sequential assignments of α-synuclein. Biomol NMR Assign 6:51–55
Gath J, Bousset L, Habenstein B et al (2014a) Yet another polymorph of alpha-synuclein: solid-state sequential assignments. Biomol NMR Assign 8:395–404
Gath J, Bousset L, Habenstein B et al (2014b) Unlike twins: an NMR comparison of two α-synuclein polymorphs featuring different toxicity. PLoS ONE 9:1–11
Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY (2001) A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem 276:2380–2386
Gopinath T, Nelson SED, Soller KJ, Veglia G (2017) Probing the conformationally excited states of membrane proteins via 1 H-detected MAS solid-state NMR spectroscopy. J Phys Chem B 121:4456–4465
Grzesiek S, Bax A (1992a) Improved 3D triple-resonance NMR techniques applied to a 31 kDa protein. J Magn Reson 96:432–440
Grzesiek S, Bax A (1992b) Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J Am Chem Soc 114:6291–6293
Guerrero-Ferreira R, Taylor NM, Mona D et al (2018) Cryo-EM structure of alpha-synuclein fibrils. Elife 7:e36402
Hardy EH, Verel R, Meier BH (2001) Fast MAS total through-bond correlation spectroscopy. J Magn Reson 148:459–464
Heise H, Hoyer W, Becker S et al (2005) Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci USA 102:15871–15876
Keller R (2004) The computer aided resonance assignment tutorial, first. CANTINA Verlag, Goldau
Li B, Ge P, Murray KA et al (2018a) Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun 9:1–10
Li Y, Zhao C, Luo F et al (2018b) Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res 28:897–903
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Mayer LD, Hope MJ, Cullis PR (1986) Vesicles of variable sizes produced by a rapid extrusion procedure. BBA 858:161–168
Morcombe CR, Zilm KW (2003) Chemical shift referencing in MAS solid state NMR. J Magn Reson 162:479–486
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Murphy DD, Rueter SM, Trojanowski JQ, Lee VM (2000) Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci 20:3214–3220
Ryan T, Bamm VV, Stykel MG et al (2018) Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat Commun 9:817
Shavali S, Brown-Borg HM, Ebadi M, Porter J (2008) Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett 439:125–128
Siemer AB, Arnold AA, Ritter C et al (2006) Observation of highly flexible residues in amyloid fibrils of the HET-s prion. J Am Chem Soc 128:13224–13228
Su LJ, Auluck PK, Outeiro TF et al (2010) Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson’s disease models. Dis Model Mech 3:194–208
Theillet FX, Binolfi A, Bekei B et al (2016) Structural disorder of monomeric alpha-synuclein persists in mammalian cells. Nature 530:45–50
Tuttle MD, Comellas G, Nieuwkoop AJ et al (2016) Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat Struct Mol Biol 23:409–415
Ulmer TS, Bax A, Cole NB, Nussbaum RL (2005) Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem 280:9595–9603
Viennet T, Wördehoff MM, Uluca B et al (2018) Structural insights from lipid-bilayer nanodiscs link α-Synuclein membrane-binding modes to amyloid fibril formation. Commun Biol 1:1–12
Wang Y, Jardetzky O (2002) Probability-based protein secondary structure identification using combined NMR chemical-shift data. Protein Sci 11:852–861
Ward ME, Ritz E, Ahmed MA et al (2015) Proton detection for signal enhancement in solid-state NMR experiments on mobile species in membrane proteins. J Biomol NMR 63:375–388
Watson JB, Hatami A, David H et al (2009) Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience 159:501–513
Zhou DH, Rienstra CM (2008) High-performance solvent suppression for proton detected solid-state NMR. J Magn Reson 192:167–172
Acknowledgements
We thank Ms. Paula Boubel for her assistance with collecting initial MAS NMR data, and Dr. Sameer Al-Abdul-Wahid of the University of Guelph NMR Centre for help with setting 3D NMR experiments. We are grateful to Prof. Alfonso De Simone (Imperial College London) for sharing chemical shift data for α-syn.
Accession numbers
Chemical shifts for assigned residues in the sample of α-syn bound to DOPA:DOPC liposomes have been deposited to BioMagResBank (http://www.bmrb.wisc.edu) under BMRB entry 50746.
Funding
This research was supported by Natural Science ang Engineering and Research Council of Canada (Discovery Grant RGPIN-2014-04547 to V.L.; Discovery Grant RGPIN-121541 to G.H.) and by the Parkinson Foundation of Canada (Grant 2018-00285 to V.L.) Canadian Institutes for Health Research Project Grant (PJT-159443-2018) to S.D.R, Canada Foundation for Innovation and Ontario Ministry of Economic Development and Innovation, and by the University of Guelph through their support of the Advanced Analysis Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medeiros, J., Bamm, V.V., Jany, C. et al. Partial magic angle spinning NMR 1H, 13C, 15N resonance assignments of the flexible regions of a monomeric alpha-synuclein: conformation of C-terminus in the lipid-bound and amyloid fibril states. Biomol NMR Assign 15, 297–303 (2021). https://doi.org/10.1007/s12104-021-10020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-021-10020-z