Abstract
Objective(s)
Exosomal HER2 has been evidenced to interfere with antibody-induced anti-tumor effects. However, whether the blockade of HER2+ exosomes release would affect antibody-mediated tumor inhibition has yet to be investigated.
Methods
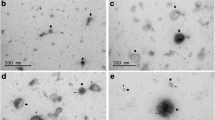
Exosomes derived from BT-474, SK-BR3 and SK-OV3 (HER2-overexpressing tumor cells) and MDA-MB-231 cells (HER2 negative) were purified and characterized by bicinchoninic acid (BCA) assay, western blotting and Transmission electron microscopy (TEM). Inhibition of exosome release was achieved by neutral sphingomyelinase-2 (nSMase-2) inhibitor, GW4869. The effects of exosome blockade on the anti-proliferative effects, apoptosis induction, and antibody-mediated cellular cytotoxicity (ADCC) activity of Trastuzumab were examined using MTT, flow cytometry, and LDH release assays. Also, the effects of exosome inhibition on the surface expression and endocytosis/internalization of HER2 were studied by flow cytometry.
Results
Purified exosomes derived from HER2 overexpressing cancer cells were positive for HER2 protein. Blockade of exosome release was able to significantly improve apoptosis induction, anti-proliferative and ADCC responses of Trastuzumab dose dependently. The pretreatment of Trastuzumab/purified NK cells, but not PBMCs, with HER2+ exosomes could also decrease the ADCC effects of Trastuzumab. Exosome inhibition also remarkably downregulated surface HER2 levels in a time-dependent manner, but does not affect its endocytosis/internalization.
Conclusion
Based on our findings, HER2+ exosomes may benefit tumor progression by dually suppressing Trastuzumab-induced tumor growth inhibition and cytotoxicity of NK cells. It seems that concomitant blocking of exosome release might be an effective approach for improving the therapeutic effects of Trastuzumab, and potentially other HER2-directed mAbs. In addition, the exosome secretion pathway possibly contributes to the HER2 trafficking to plasma membrane, since the blockade of exosome secretion decreased surface HER2 levels.







Similar content being viewed by others
References
Hsu JL, Hung M-C. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–88.
Gradishar WJ. HER2 therapy—an abundance of riches. N Engl J Med. 2012;366(2):176–8.
Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11(1):1–11.
Hoeferlin LA, Chalfant CE, Park MA. Challenges in the treatment of triple negative and HER2-overexpressing breast cancer. J Surg Sci. 2013;1(1):3.
Xu Z-q, Zhang Y, Li N, Liu P-j, Gao L, Gao X, et al. Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(3):e013053.
Oh D-Y, Bang Y-J. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Shimada Y, Matsubayashi J, Kudo Y, Maehara S, Takeuchi S, Hagiwara M, et al. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci Rep. 2021;11(1):7830.
Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME, et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer. 2021;20(1):83.
Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–70.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–67.
Martinez VG, O’Neill S, Salimu J, Breslin S, Clayton A, Crown J, et al. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology. 2017;6(12): e1362530.
Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–48.
Tian F, Zhang S, Liu C, Han Z, Liu Y, Deng J, et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun. 2021;12(1):1–13.
Liu C, Yang Y, Wu Y. Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS J. 2018;20(2):1–13.
Hekmatirad S, Moloudizargari M, Moghadamnia AA, Kazemi S, Mohammadnia-Afrouzi M, Baeeri M, et al. Inhibition of exosome release sensitizes U937 cells to PEGylated liposomal doxorubicin. Front Immunol. 2021;12:2008.
Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414-27. e13.
Schneider E, Winzer R, Rissiek A, Ricklefs I, Meyer-Schwesinger C, Ricklefs FL, et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat Commun. 2021;12(1):1–14.
Hosseini-Ghatar R, Soltantoyeh T, Bahadori M, Golara M, Hassannia H, Khosravi-Eghbal R, et al. Epitope mapping of human HER2 specific mouse monoclonal antibodies using recombinant extracellular subdomains of HER2. Asian Pac J Cancer Prev. 2017;18(11):3103–10.
Hosseini-Ghatar R, Soltantoyeh T, Bahadori M, Khoshnoodi J, Golsaz-Shirazi F, Jeddi-Tehrani M, et al. Polyclonal antibody against different extracellular subdomains of HER2 induces tumor growth inhibition in vitro. Iran J Immunol. 2017;14(3):200–14.
Hassannia H, Amiri MM, Jadidi-Niaragh F, Hosseini-Ghatar R, Khoshnoodi J, Sharifian R-A, et al. Inhibition of tumor growth by mouse ROR1 specific antibody in a syngeneic mouse tumor model. Immunol Lett. 2018;193:35–41.
Yu S-F, Zheng B, Go M, Lau J, Spencer S, Raab H, et al. A novel anti-CD22 anthracycline-based antibody–drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21(14):3298–306.
Nejadmoghaddam M-R, Zarnani A-H, Ghahremanzadeh R, Ghods R, Mahmoudian J, Yousefi M, et al. Placenta-specific1 (PLAC1) is a potential target for antibody-drug conjugate-based prostate cancer immunotherapy. Sci Rep. 2017;7(1):1–13.
Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova I-I. Therapy resistance mediated by exosomes. Mol Cancer. 2019;18(1):58.
Hayatudin R, Fong Z, Ming LC, Goh B-H, Lee W-L, Kifli N. Overcoming chemoresistance via extracellular vesicle inhibition. Front Mol Biosci. 2021;8:158.
Yang Y, Li C-W, Chan L-C, Wei Y, Hsu J-M, Xia W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4.
Chew HY, De Lima PO, Cruz JLG, Banushi B, Echejoh G, Hu L, et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell. 2020;180(5):895-914. e27.
Gall VA, Philips AV, Qiao N, Clise-Dwyer K, Perakis AA, Zhang M, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Can Res. 2017;77(19):5374–83.
Wymant JM, Sayers EJ, Muir D, Jones AT. Strategic trastuzumab mediated crosslinking driving concomitant HER2 and HER3 endocytosis and degradation in breast cancer. J Cancer. 2020;11(11):3288.
Paris L, Cecchetti S, Spadaro F, Abalsamo L, Lugini L, Pisanu ME, et al. Inhibition of phosphatidylcholine-specific phospholipase C downregulates HER2 overexpression on plasma membrane of breast cancer cells. Breast Cancer Res. 2010;12(3):1–16.
Koay DC, Zerillo C, Narayan M, Harris LN, DiGiovanna MP. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res. 2010;12(4):1–19.
Liu Z, Sang X, Wang M, Liu Y, Liu J, Wang X, et al. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene. 2021;40(44):6273–83.
She Q-B, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3(8): e3065.
Menck K, Sönmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles. 2017;6(1):1378056.
Back MJ, Ha HC, Fu Z, Choi JM, Piao Y, Won JH, et al. Activation of neutral sphingomyelinase 2 by starvation induces cell-protective autophagy via an increase in Golgi-localized ceramide. Cell Death Dis. 2018;9(6):1–18.
Hao M, Yeo SK, Turner K, Harold A, Yang Y, Zhang X, et al. Autophagy blockade limits HER2+ breast cancer tumorigenesis by perturbing HER2 trafficking and promoting release via small extracellular vesicles. Dev Cell. 2021;56(3):341-55. e5.
Zhang J, Fan J, Zeng X, Nie M, Chen W, Wang Y, et al. Targeting the autophagy promoted antitumor effect of T-DM1 on HER2-positive gastric cancer. Cell Death Dis. 2021;12(4):1–14.
Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Can Res. 2001;61(12):4892–900.
Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci. 2011;108(37):15336–41.
Collins D, O’donovan N, McGowan P, O’sullivan F, Duffy M, Crown J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. 2012;23(7):1788–95.
Collins DM, Madden SF, Gaynor N, AlSultan D, Le Gal M, Eustace AJ, et al. Effects of HER family–targeting tyrosine kinase inhibitors on antibody-dependent cell-mediated cytotoxicity in HER2-expressing breast cancer. Clin Cancer Res. 2021;27(3):807–18.
Collins DM, Gately K, Hughes C, Edwards C, Davies A, Madden SF, et al. Tyrosine kinase inhibitors as modulators of trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity in breast cancer cell lines. Cell Immunol. 2017;319:35–42.
Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, et al. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. 2022;21(1):1–18.
Ganau M. Tackling gliomas with nanoformulated antineoplastic drugs: suitability of hyaluronic acid nanoparticles. Clin Transl Oncol. 2014;16(2):220–3.
Ragni E, PeruccaOrfei C, De Luca P, Lugano G, Viganò M, Colombini A, et al. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res Ther. 2019;10(1):1–17.
Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875-IN4.
Greenberg JW, Kim H, Moustafa AA, Datta A, Barata PC, Boulares AH, et al. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci Rep. 2021;11(1):1–12.
Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep. 2018;8(1):1–13.
Acknowledgements
This study was supported by grants from Isfahan University of Medical Sciences, Isfahan, Iran (grant number#51435); and Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and Informed consent
It should be noted that for PBMCs isolation, a signed consent letter was taken from healthy donors (n = 3), and all the protocols of this study were approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.569).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, R., Asef-Kabiri, L., Sarvnaz, H. et al. Blockade of exosome release alters HER2 trafficking to the plasma membrane and gives a boost to Trastuzumab. Clin Transl Oncol 25, 185–198 (2023). https://doi.org/10.1007/s12094-022-02925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02925-5