Abstract
Purpose
The activation of stimulator of interferon genes (STING) pathway triggers the antitumor immunity by CD8 + T cells. However, the differentiated antitumor effects of STING activation in different cell types is still unclear. We aimed to investigate the expression and potential prognostic value of cancer cell-intrinsic STING in hepatocellular carcinoma (HCC), and whether STING could be a potential immunotherapeutic target of HCC was then evaluated.
Methods
We separately assessed the expression of STING in cancer cells and infiltrating immune cells in HCC tissues. The independent clinicopathological factors associated with survival outcomes were evaluated by the multivariable analysis. The HCC orthotopic mice model were used to confirm the immunotherapeutic effects of STING agonists, and CD8 + T-cell infiltration level was analyzed through immunofluorescence and flow cytometry.
Results
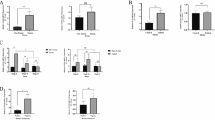
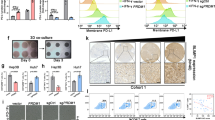
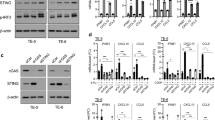
The expression of cancer cell-intrinsic STING was significantly reduced in HCC compared with adjacent tissues. Patients with low levels of cancer cell-intrinsic STING expression was associated with increased tumor volume (P = 0.009), higher serum AFP levels (P = 0.028), and decreased CD8 + T-cell infiltration (P = 0.002). Low levels of cancer cell-intrinsic STING expression indicated a poor overall survival (OS) and disease-free survival (DFS). Multivariate analysis demonstrated that low levels of cancer cell-intrinsic STING expression was an independent prognostic factor. Additionally, cancer cell-intrinsic STING expression was positively related with CD8 + T-cell infiltration levels in HCC patients (r = 0.308; P = 0.001). When mice with orthotopic HCC tumors treated with STING agonists, tumor growth was significantly reduced with enhanced levels of CD8 + T-cell infiltration.
Conclusion
Cancer cell-intrinsic STING might affect HCC tumor progression through enhancing CD8 + T-cell infiltration and can be an immunotherapeutic target for HCC.




Similar content being viewed by others
References
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62. https://doi.org/10.1056/NEJMra1713263.
Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship With clinical and pathological features. Hepatology. 2016;64(6):2038–46. https://doi.org/10.1002/hep.28710.
Fang Q, Xie QS, Chen JM, Shan SL, Xie K, Geng XP, et al. Long-term outcomes after hepatectomy of huge hepatocellular carcinoma: a single-center experience in China. Hepatobiliary Pancreat Dis Int. 2019;18(6):532–7. https://doi.org/10.1016/j.hbpd.2019.09.001.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. https://doi.org/10.1038/s41571-018-0073-4.
Khoo LT, Chen LY. Role of the cGAS-STING pathway in cancer development and oncotherapeutic approaches. EMBO Rep. 2018. https://doi.org/10.15252/embr.201846935.
Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18(1):152. https://doi.org/10.1186/s12943-019-1087-y.
Tao J, Zhou X, Jiang Z. cGAS-cGAMP-STING: the three musketeers of cytosolic DNA sensing and signaling. IUBMB Life. 2016;68(11):858–70. https://doi.org/10.1002/iub.1566.
Ng KW, Marshall EA, Bell JC, Lam WL. cGAS-STING and cancer: dichotomous roles in tumor immunity and development. Trends Immunol. 2018;39(1):44–54. https://doi.org/10.1016/j.it.2017.07.013.
Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Investig. 2019;129(3):1211–28. https://doi.org/10.1172/jci123319.
Chon HJ, Kim H, Noh JH, Yang H, Lee WS, Kong SJ, et al. STING signaling is a potential immunotherapeutic target in colorectal cancer. J Cancer. 2019;10(20):4932–8. https://doi.org/10.7150/jca.32806.
Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig. 2019a;130(10):4350–64. https://doi.org/10.1172/jci125413.
Falahat R, Perez-Villarroel P, Mailloux AW, Zhu G, Pilon-Thomas S, Barber GN, et al. STING signaling in melanoma cells shapes antigenicity and can promote antitumor T-cell activity. Cancer Immunol Res. 2019;7(11):1837–48. https://doi.org/10.1158/2326-6066.cir-19-0229.
Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2019;29(3):785–9. https://doi.org/10.1016/j.celrep.2019.09.089.
Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36(4):250–6. https://doi.org/10.1016/j.it.2015.02.003.
Sokolowska O, Nowis D. STING signaling in cancer cells: important or not? Arch Immunol Ther Exp. 2018;66(2):125–32. https://doi.org/10.1007/s00005-017-0481-7.
Ranoa DRE, Widau RC, Mallon S, Parekh AD, Nicolae CM, Huang X, et al. STING promotes homeostasis via regulation of cell proliferation and chromosomal stability. Cancer Res. 2019;79(7):1465–79. https://doi.org/10.1158/0008-5472.can-18-1972.
Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7:39858. https://doi.org/10.1038/srep39858.
Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14(2):282–97. https://doi.org/10.1016/j.celrep.2015.12.029.
Konno H, Yamauchi S, Berglund A, Putney RM, Mulé JJ, Barber GN. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene. 2018;37(15):2037–51. https://doi.org/10.1038/s41388-017-0120-0.
Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig. 2019b;130:4350–64. https://doi.org/10.1172/jci125413.
Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194(7):3475–86. https://doi.org/10.4049/jimmunol.1402711.
Trujillo JA, Sweis RF, Bao R, Luke JJ. T Cell-Inflamed versus Non-T cell-inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res. 2018;6(9):990–1000. https://doi.org/10.1158/2326-6066.cir-18-0277.
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics (Oxford, England). 2019;35(20):4200–2. https://doi.org/10.1093/bioinformatics/btz210.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–10. https://doi.org/10.1158/0008-5472.can-17-0307.
Thomsen MK, Skouboe MK, Boularan C, Vernejoul F, Lioux T, Leknes SL, et al. The cGAS-STING pathway is a therapeutic target in a preclinical model of hepatocellular carcinoma. Oncogene. 2020;39(8):1652–64. https://doi.org/10.1038/s41388-019-1108-8.
Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2018;25(11):3074-85.e5. https://doi.org/10.1016/j.celrep.2018.11.047.
Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA. 2015;112(50):15408–13. https://doi.org/10.1073/pnas.1512832112.
Corrales L, McWhirter SM, Dubensky TW Jr, Gajewski TF. The host STING pathway at the interface of cancer and immunity. J Clin Investig. 2016;126(7):2404–11. https://doi.org/10.1172/jci86892.
Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–61. https://doi.org/10.1158/2159-8290.cd-18-1020.
Pantelidou C, Sonzogni O, De Oliveria TM, Mehta AK, Kothari A, Wang D, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral sting pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9(6):722–37. https://doi.org/10.1158/2159-8290.cd-18-1218.
Liang D, Xiao-Feng H, Guan-Jun D, Er-Ling H, Sheng C, Ting-Ting W, et al. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim Biophys Acta. 2015;1852(11):2494–503. https://doi.org/10.1016/j.bbadis.2015.08.011.
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76(8):2076–81. https://doi.org/10.1158/0008-5472.can-15-1456.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. https://doi.org/10.1016/j.it.2016.01.002.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–52. https://doi.org/10.1016/j.immuni.2018.03.014.
Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–82. https://doi.org/10.1038/nri3902.
Funding
This study was supported by grants from the Innovative Research Groups of National Natural Science Foundation of China (No. 81721091), the National Natural Science Foundation of China (No.81901237), and Health Commission of Zhejiang Province (JBZX-202004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the concept and design of the study. YZ and QZ performed samples collection, data analysis, and the writing of this study. YZ, XF, DC, YL and JH constructed the animal models and performed flow cytometry. HX conducted IHC and IF study, LZ and JW conducted WB and all other studies. SZ supervised the process of the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirmed no conflicts of interest in this work.
Ethical approval
Approval was obtained from the ethics committee of Zhejiang University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhai, Q., Feng, X. et al. Cancer cell-intrinsic STING is associated with CD8 + T-cell infiltration and might serve as a potential immunotherapeutic target in hepatocellular carcinoma. Clin Transl Oncol 23, 1314–1324 (2021). https://doi.org/10.1007/s12094-020-02519-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02519-z