Abstract
Background
With 9.6 million deaths in 2018, cancer remains the second leading cause of death worldwide. Breast cancer is the most deadly type of cancer among females, with 55.2% of crude incidence rate and 16.6% of crude mortality rate.
Purpose
The present study was aimed to investigate the anti-breast cancer potential of natural dietary flavonoid, apigenin isolated from Clerodendrum viscosum leaves.
Methods
Apigenin was evaluated for in-depth anticancer activity in MCF-7 cells using cell viability assay, cell cycle analysis, Annexin-V-FLUOS staining, ROS induction, morphological analysis, and western blot analysis.
Results
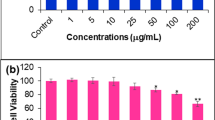
Apigenin showed selective cytotoxicity on MCF-7 cells with an IC50-56.72 ± 2.35 µM, while negligible cytotoxicity was observed on WI-38 cells. Further, the flow cytometer-based analysis showed that apigenin halted MCF-7 cells in the G2/M phase arrest followed by dose-dependent apoptosis. Moreover, the FACS and confocal microscopy results confirmed the elevation of intracellular ROS and nuclear fragmentation in apigenin-treated MCF-7 cells. Western blots showed up-regulation of cell cycle regulatory proteins, increased p53 expression, Bax/Bcl-2 ratio, activation of caspases, and cleavage of PARP. Finally, apigenin treatment in the presence of Pifithrin-µ showed decreased apoptotic population and it was further confirmed through western blotting study. The results revealed the vital role of p53 in apigenin-induced apoptosis in MCF-7 cells.
Conclusions
In the present findings, treatment of apigenin-induced intracellular ROS in MCF-7 cells followed by induction of G2/M phase cell cycle arrest and further apoptosis through the regulation of p53 and caspase-cascade signaling pathway.









Similar content being viewed by others
References
World Health Organization. Global Cancer Observatory. International agency for research on cancer. 2019. https://gco.iarc.fr. Accessed 05 Nov 2019.
Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116.
Itoh Y, Sendo T, Hirakawa T, Goromaru T, TakasakibS YH, et al. Role of sensory nerve peptides rather than mast cell histamine in paclitaxel hypersensitivity. Am J Respir Crit Care Med. 2004;169:113–9.
Wilson CR, Sauer JM, Hooser SB. Taxines: a review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon. 2001;39:175–85.
Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by Paclitaxel: recent insights and future perspectives. Curr neuropharmacol. 2006;4:165–72.
Safra T, Menczer J, Bernstein RM, Shpigel S, Matcejevsky D, Inbar MJ, et al. Combined weekly carboplatin and paclitaxel as primary treatment of advanced epithelial ovarian carcinoma. Gynecol Oncol. 2009;114:215–8.
Ajani JA, Dodd LG, Daugherty K, Warkentin D, Ilson DH. Taxol induced soft-tissue injury secondary to extravasation: characterization by histopathology and clinical course. J Natl Cancer Inst. 1994;86:51–3.
Griesbach R. Biochemistry and genetics of flower color. Plant Breed Rev. 2005;25:89–114.
Das AD, Chaudhuri D, Ghate NB, Chatterjee A, Mandal N. Comparative assessment of phytochemicals and antioxidant potential of methanolic and aqueous extracts of Clerodendrum colebrookianum walp. leaf from north-east India. Int J Pharm Pharm Sci. 2013;5:420–7.
Srivasatava N, Patel T. Clerodendrum and health care: an overview. Med Aromat Plant Sci Biotechnol. 2007;1:142–50.
Dey P, Chaudhuri D, Tamang S, Chaudhuri TK, Mandal N. In vitro antioxidant and free radical scavenging potential of Clerodendrum viscosum. Int J Pharm Bio Sci. 2012;3:454–71.
Shendge AK, Basu T, Chaudhuri D, Panja S, Mandal N. In Vitro antioxidant and antiproliferative activities of various solvent fractions from Clerodendrum viscosum leaves. Pharmacogn Mag. 2017;13:344–53.
Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, et al. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0082293.
Ovando C, Hernandez D, Hernandez E, et al. Chemical studies of anthocyanins: a review. Food Chem. 2009;113:859–71.
Lee Y, Yuk D, Lee J, et al. Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of β-amyloid generation and memory deficiency. Brain Res. 2009;1250:164–74.
Romanova D, Vachalkova A, Cipak L, Ovesna Z, Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48:104–7.
Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A. The Therapeutic Potential of Apigenin. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20061305.
Wang B, Zhao XH. Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma HCT-116 cells. Oncol Rep. 2017;37:1132–40.
Souza RP, Bonfim-Mendonca PS, Gimenes F, et al. Oxidative stress triggered by apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid Med Cell Longev. 2017. https://doi.org/10.1155/2017/1512745.
Choi SI, Jeong CS, Cho SY, Lee YS. Mechanism of apoptosis induced by apigenin in HepG2 human hepatoma cells: involvement of reactive oxygen species generated by NADPH oxidase. Arch Pharm Res. 2007;30:1328–35.
Jung HA, Chung HY, Yokozawa T, Kim YC, Hyun SK, Choi JS. Alaternin and emodin with hydroxyl radical inhibitory and/or scavenging activities and hepatoprotective activity on tacrine-induced cytotoxicity in HepG2 cells. Arch Pharm Res. 2004;27:947–53.
Jing X, Ueki N, Cheng J, Imanishi H, Hada T. Induction of apoptosis in hepatocellular carcinoma cell lines by emodin. Jpn J Cancer Res. 2002;93:874–82.
Yi J, Yang J, He R, et al. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–16.
Yang J, Li H, Chen YY, et al. Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species-mediated dual regulation of apoptosis. Free Radic Biol Med. 2004;37:2027–41.
Tavsan Z, Kayali HA. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed Pharmacother. 2019. https://doi.org/10.1016/j.biopha.2019.109004.
Vrhovac Madunic I, Madunic J, Antunovic M, et al. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:537–50.
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118–24.
Darzynkiewicz Z, Huang X. Analysis of cellular DNA content by flow cytometry. Curr Protoc Immunol. 2004. https://doi.org/10.1002/0471142735.im0507s60.
Vermes I, Haanen C, Steffens-Nakken H. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51.
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016. https://doi.org/10.1155/2016/4350965.
Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J Biolog Chem. 2002;277:17179–877.
Marullo R, Werner E, Degtyareva N, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetics functions. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0081162.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment - could arsenic have a role. J Ovar Res. 2009. https://doi.org/10.1186/1757-2215-2-2.
Jing Y, Yang J, Wang Y, et al. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor kappaB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med. 2006;40:2183–97.
Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52.
Jing YW, Yi J, Chen YY, et al. Dicoumarol alters cellular redox state and inhibits nuclear factor kappaB to enhance arsenic trioxide-induced apoptosis. Acta Biochim Biophys Sin (Shanghai). 2004;36:235–42.
Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–9.
Wang Y, Ji P, Liu J, Broaddus RR, Xue F, Zhang W. Centrosome-associated regulators of the G2/M checkpoint as targets for cancer therapy. Mol Cancer. 2009. https://doi.org/10.1186/1476-4598-8-8.
Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–34.
Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016;42:63–71.
Choi EJ, Kim GH. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J Nutr Biochem. 2009;20:285–90.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10.
Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–85.
Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–500.
Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33.
Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007. https://doi.org/10.1186/bcr1797.
Acknowledgements
AKS and DC are grateful to the Council of Scientific and Industrial Research (CSIR) Govt. of India and Tapasree Basu is grateful to the University Grants Commission (UGC), Govt. of India for providing the fellowships. The authors are thankful to Dr. Nikhil Baban Ghate for his constant guidance during the experiments and Mr. Ranjit Kumar Das for his technical assistance.
Funding
This research did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AKS, DC, TB, and NM declared no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Informed consent
The manuscript does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shendge, A.K., Chaudhuri, D., Basu, T. et al. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin Transl Oncol 23, 718–730 (2021). https://doi.org/10.1007/s12094-020-02461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02461-0