Abstract
Objective
To compare the efficiency of adjuvant therapy with aromatase inhibitors or with tamoxifen in postmenopausal women with operable breast cancer and positive estrogen receptors.
Material and methods
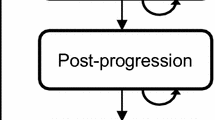
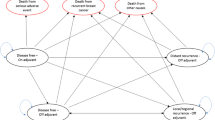
A cost-utility analysis was performed based on a Markov model, from the Spanish National Health Care System perspective, comparing the treatment with exemestane (EXE: 25 mg/day) or tamoxifen (TAM: 20 mg/day) after 2–3 years of monotherapy with TAM; anastrozole (ANA, 1 mg/day) or TAM (20 mg/day) without previous TAM therapy; and letrozole (LET: 2.5 mg/day) or placebo after 5 years of monotherapy with TAM. The follow-up of a hypothetical cohort of women starting treatment at 63 years of age was simulated during 10 and 20 years. The probabilities of transition between health states and quality adjusted life years (QALYs) were obtained from the literature, and the unit costs (∈ corresponding to 2004) from a Spanish database.
Results
After 10 and 20 years of follow-up, more QALYs per patient would be gained with the EXE scheme (0.230–0.286 and 0.566–0.708, respectively) than with ANA (0.114 and 0.285) and LET (0.176 and 0.474). The cost of gaining one QALY was lower with the EXE scheme (50,801–62,522 ∈ and 28,849–35,371 ∈ respectively) than with ANA (104,272 ∈ and 62,477 ∈) and LET (91,210 ∈ and 49,460 ∈). The result was stable for the cost per life-year gained (LYG) and in the sensitivity analysis.
Conclusions
The EXE scheme after TAM is more cost-effective than the ANA and LET schemes.
Similar content being viewed by others
References
OncoGuía de mama. Agencia de Evaluación de Tecnologias e Investigaciones Médicas. CatSalut. Departamento de Sanidad y Seguridad Social. Generalidad de Cataluña. Barcelona: noviembre 2003 (OG04/2005).
Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896; 2:104–7.
Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2005;9:1980–9.
Early Breast Cancer Trialists' Collaborative Group (EBCTG). Effects of chemotherapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365: 1687–717.
Ligibel JA, Winer EP. Clinical differences among the aromatase inhibitors. Clin Cancer Res. 2003;9:4755–95.
Coombes RC, Hall E, Gibson LJ, et al for the Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;550:1081–92.
Coombes RC, Hall E, Snowdon CF, Bliss JM. A randomized trial in postmenopausal patients with early breast cancer who remain disease-free after two to three years of tamoxifen. Intergroup Exemestane Study. Updated survival analysis 27th Annual San Antonio Breast Cancer Symposium. General Session 1. December 8, 2004. Available from: http://www.abstracts2view.com/sabcs/search.php?search =do&intMaxHits=10&where%5B%5D=&andornot%5B%5D=&query=coombes (consulta: mayo de 2005).
The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists' Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2151–9.
Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;549:1793–802.
Gisbert R, Brosa M. Base de datos de costes sanitarios. Versión 1.5. Barcelona: Soikos, 2004.
Base de datos de medicamentos. Consejo General de Colegios Oficiales de Farmacéuticos. Disponible en URL: http://www. portalfarma.com/home.nsf (consulta: octubre, 2004).
Rubio-Terrés C, Sacristán JA, Badía X, Cobo E, García Alonso F, por el Grupo ECOMED. Metodos utilizados para realizar evaluaciones económicas de intervenciones sanitarias. Med Clin. 2004;122: 578–85.
Rubio Terrés C. Introducción a la utilización de los modelos de Markov en el análisis farmacoeconómico. Farm Hosp. 2000; 24:241–7.
DATA, 3.5 for Healthcare. Williamstown (MA): TreeAge Software Inc, 1999.
Hillner BE. Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer. 2004;101:1311–22.
Simons WR, Jones D, Buzdar A. Cost-effectiveness of anastrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer. Clin Ther. 2003;25:2972–87.
Skedgel C, Rayson D, Dewar R, Potvin K, Younis T. Economic evaluation of adjuvant hormonal options in postmenopausal women with breast cancer: Tamoxifen vs tamoxifen then exemestane vs anastrozole. 2005 ASCO Annual Meeting. Abstract 6040. Available from: http://www.asco.org/ ac/1,1005_12-002948-00_18-0058906,00.asp (consulta: mayo 2005).
Pérez EA, Mincey BA. Hormonal treatment of breast cancer in the extended adjuvant setting (mayo 2004). Available from: http://www.medscape.com/viewprogram/ 5082_pnt (consulta: mayo de 2005).
Lonning PE. Aromatase inhibitors in breast cancer. Endocrine-related Cancer. 2004;11:179–89.
Smith IE, Adjuvant hormonal therapy for breast cancer: results of the major trials. In: Smith IE, Thuerlimann B, Adjuvant Hormonal Therapy for Breast Cancer: An Evolving Process (Archived Web Conference) (enero 2005). Available from: http://www.medscape.com/viewprogram/ 3800_pnt (consulta: mayo de 2005).
Campos SM. Aromatase inhibitors for breast cancer in postmenopausal women. The Oncologist. 2004;9:126–56.
Gradishar WJ. Tamoxifen—What next? The Oncologist. 2004;9:378–84.
Annual Meetings of the American Society of Clinical Oncology. http://www.asco.org (consulta: mayo de 2005).
Annual San Antonio Breast Cancer Symposiums. http://www.sabcs.org (consulta: mayo de 2005).
Aromatase Inhibitors in the Adjuvant Setting: Updates on ATAC, MA-17, and IES. Fourth European Breast Cancer Conference March 16–20, 2004, Hamburg, Germany. Available from: http://www. medscape. com/viewarticle/474061? scr=search (consulta: mayo de 2005).
Centro Nacional de Epidemiologia. Mortalidad por causa, sexo y grupo de edad (CIE6). España, 1998. Disponible en URL: http://195.146.50.150. (consulta: mayo, 2005).
NICE. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence, April 2004.
INEbase. Tablas de mortalidad de la población de España 1998–1999. Datos nacionales. Disponible en URL: http:// www.ine.es/inebase/cgi/axi (consulta: mayo, 2005).
Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis. Methods for quantitative synthesis in medicine. New York: Oxford University Press: 1994.
Rovira J, Antoñanzas F. Economic analysis of health technologies and programmes. A Spanish proposal for methodological standardisation. Pharmacoeconomics. 1995;8:245–52.
Canadian Coordinating Office for Health Technology Assessment. Guideline for economic evaluation of pharmaceuticals: Canada. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA), 1997.
Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on good research practices-modeling studies. Value Health. 2003;6:9–17.
Ficha técnica: Aromasil (Exemestano). Pharmacia Spain, SA, Septiembre de 2004.
Ficha técnica: Arimidex (Anastrozol). Astra-Zeneca Farmacéutica Spain, SA. Julio de 2004.
Ficha técnica: Femara (Letrozol). Novartis Farmacéutica, SA. Febrero de 2005.
Ficha técnica: Nolvadex (Tamoxifeno). AstraZeneca Farmacéutica Spain, SA. Septiembre de 2003.
Albaina L, Viana C. Guias Clinicas de Atención Primaria: Cáncer de mama. Guías Clínicas 2003; 3 (7). Disponible en URL: http://www.fisterra.com/guias2/mama. asp (consulta: abril de 2005).
Karnon J, Brown J. Adjuvant Breast Cancer (ABC) Steering Committee, Tamoxifen plus chemotherapy versus tamoxifen alone as adjuvant therapies for node-positive postmenopausal women with early breast cancer: a stochastic economic evaluation. Pharmacoeconomics. 2002;20:119–57.
Gabriel SE, Kneeland TS, Melton LJ 3rd, Moncur MM, Ettinger B, Tosteson AN. Health-related quality of life in economic evaluations for osteoporosis: whose values should we use? Med Decis Making. 1999;19:141–8.
Brunner HI, Chan W-S, Ginsberg JS, Feldman BM. Longterm anticoagulation is preferable for patients with antiphospholipid antibody syndrome. Result of a decision analysis. Disponible en URL: http: //www.jrheum.com/suscribers/02/03/tables/490-3.html (consulta: mayo de 2005).
Nicholson T, McGuire A, Milne R. Cost-utility of enoxaparin compared with unfractionated heparin in unstable coronary artery disease. BMC Cardiovasc Disord. 2001;1:2.
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. Qué es una tecnología sanataria eficiente en España. Gac Sanit. 2002; 16:334–43.
Plans Rubió P. Cost-effectiveness of pharmacologic treatments for the reduction of blood lipids. Med Clin (Barc). 1995;105: 327–33.
Plans-Rubió P. Cost-effectiveness of cardiovascular prevention programs in Spain. Int J Technol Assess Health Care. 1998; 14:320–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gil, J.M., Rubio-Terrés, C., del Castillo, A. et al. Pharmacoeconomic analysis of adjuvant therapy with exemestane, anastrozole, letrozole or tamoxifen in postmenopausal women with operable and estrogen receptor-positive breast cancer. Clin Transl Oncol 8, 339–348 (2006). https://doi.org/10.1007/s12094-006-0180-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12094-006-0180-z