Abstract
Objectives
In Sweden, breast cancer (BC) represents 30% of newly diagnosed cancers and is the most common cancer in women. For hormone-dependent BC, endocrine therapies varying in efficacy and price are available. The aim of this study is to assess the cost effectiveness of fulvestrant 500 mg as a second-line hormonal therapy for postmenopausal women with estrogen receptor-positive metastatic or locally advanced BC versus letrozole, anastrozole, and exemestane in Sweden.
Methods
A three-state (pre-progression, post-progression, and death) partitioned-survival model was used to estimate progression-free (PFS) and overall survival (OS) by extrapolating trial results beyond the trial period to capture costs and benefits over a lifetime perspective. The comparative effectiveness was sourced from a network meta-analysis. The evaluation was conducted from a Swedish national payer perspective; costs, resource use, and quality of life were based on published sources and expert opinion.
Results
Compared to anastrozole, letrozole, and exemestane the incremental cost-effectiveness ratios (ICERs) were €33,808, €33,883, and €49,225 per QALY with incremental costs of €13,283, €14,986, and €13,862, and incremental QALYs of 0.393, 0.442, and 0.282, respectively. Incremental cost per life-year (LY) gained €21,312 (incremental LY of 0.623), €20,338 (incremental LY of 0.737), and €27,854 (incremental LY of 0.498) for respective comparators. Applying the upper and lower credible intervals for PFS/OS from the meta-analysis had the greatest effect on the ICER in the sensitivity analysis. The results were relatively stable when varying other parameters.
Conclusions
Our results indicate that fulvestrant 500 mg may be a cost-effective alternative to aromatase inhibitors at a threshold of €100,000/QALY.
Similar content being viewed by others
A variety of endocrine therapies (ETs) are needed for advanced and metastatic breast cancer (BC) in order to meet patients’ individual needs. |
Based on a recent network meta-analysis combined with health economic modelling, fulvestrant 500 mg brings additional health gains at additional costs compared to anastrozole, letrozole, and exemestane. |
At a willingness-to-pay per quality-adjusted life-year of €100,000, the probability of fulvestrant 500 mg being cost effective is 70% compared to aromatase inhibitors in Swedish postmenopausal women with estrogen receptor-positive, locally advanced, or metastatic BC who relapse during or after previous ET. |
1 Introduction
In Sweden, breast cancer (BC) represents 30% of all newly diagnosed cancer cases [1], making it the most common type of cancer in women [2, 3]. The survival of patients with metastatic BC in Sweden has slightly improved over time, yet approximately 1500 women die from BC every year, the majority with metastatic disease [2].
Postmenopausal women who present with estrogen receptor-positive (ER+) advanced BC (ABC) are often treated with various endocrine therapies (ETs) that are generally effective and well-tolerated [2, 4, 5]. In clinical practice, several lines of ET are used for as long as the tumor remains endocrine sensitive to delay disease progression and the need for chemotherapy [4, 6, 7]. Due to lack of other predictive biomarkers, it is impossible to identify subgroups that benefit from ET most [8]. Hence, the optimal sequencing of ET in patients with ABC is not established. The choice of treatment is determined by clinical criteria, previous therapies and response, menopausal status, and patient preference. Therefore, a variety of ET needs to be available to meet patients’ individual needs [2]. The ETs not only differ in clinical profile but also in price, resulting in a substantial price difference between generic and patent-protected therapies. Given limited healthcare budgets and observed differences between treatments, the value for money presented as utility gained from money spent has become prominent on the agenda of payers [9]. Therefore, assessing the consequences of using alternative therapies in terms of lifetime costs and health gains is often required to inform decision making.
Several ETs are available for advanced and metastatic ER+ BC treatment. The most commonly used are tamoxifen and aromatase inhibitors (AIs), both available as generic medicines [2]. One of the available ETs is fulvestrant (Faslodex®), a selective ER degrader (SERD) whose mechanism of action is associated with down-regulation of estrogen receptor protein levels, which results in accelerated degradation of the ER protein and complete inhibition of estrogen signaling through the ER with no agonist activity [5]. Fulvestrant 500 mg is an effective and well-tolerated treatment option for patients with advanced or metastatic BC who have relapsed or progressed on previous ET. Fulvestrant 250 mg was supported by a large evidence base across a range of clinical studies demonstrating similar efficacy to tamoxifen, anastrozole, and exemestane [10,11,12,13]. The improved efficacy for fulvestrant 500 mg over fulvestrant 250 mg was demonstrated in the CONFIRM (Comparison of Faslodex™ in Recurrent Metastatic Breast Cancer) study. The study showed that fulvestrant 500 mg offers a significantly longer progression-free survival (PFS) than fulvestrant 250 mg [hazard ratio (HR) = 0.80 (95% confidence interval (CI) 0.68–0.94); 2-sided p = 0.006] as well as improved overall survival (OS) [HR = 0.81 (95% CI 0.69–0.96); nominal p = 0.02] [14, 15]. As a result, the recommended monthly dose in the Summary of Product Characteristics was increased from 250 to 500 mg, with an additional 500 mg dose given 2 weeks after the initial dose [16].
The aim of this study is to assess the cost effectiveness of fulvestrant 500 mg versus generic AIs as a second-line hormonal therapy in Swedish postmenopausal women with ER+ metastatic or locally advanced BC.
2 Materials and Methods
2.1 Comparators and Patient Population
The most relevant comparators for fulvestrant 500 mg in a second-line treatment in advanced ER+ BC are the AIs letrozole 2.5 mg, anastrozole 1 mg, and exemestane 25 mg.
The indicated population is postmenopausal women with ER+ locally advanced or metastatic BC, whose disease progressed or relapsed while on/after previous ET. Tamoxifen was not identified as a relevant comparator as it is commonly used as an adjuvant therapy and comes earlier in the treatment sequence.
2.2 Model
The value for money of fulvestrant 500 mg versus AIs was assessed by performing a cost-effectiveness analysis, which allows the comparison of incremental costs imposed by fulvestrant over AIs against the incremental health effects over a patient’s lifetime [9]. The health effects were expressed in quality-adjusted life-years (QALYs) calculated by estimating the total life-years (LYs) gained and by weighting the time spent in each health state by a score ranging from 0 to 1 to reflect the quality of life in that state [17]. This approach allows us to capture both quality and length of life, and is a standard framework for economic evaluations in oncology [9].
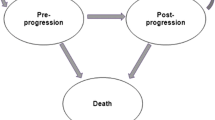
The economic analysis was conducted by using a three mutually exclusive health state model (pre-progression, post-progression, and death) which reflects the natural disease progression. To capture costs and benefits for the expected duration of the patients’ lifetime, the distribution of patients over the health states over time was estimated. Patient-level data from the CONFIRM trial [14, 15] were used to extrapolate PFS and OS for the post-trial period by fitting parametric distributions to the Kaplan–Meier (KM) data and determining the most plausible extrapolation. The chosen distributions were used to determine the distribution of patients in the pre-progression state and death state. The difference between OS and PFS curves provided the proportion of patients experiencing progressive disease.
The best fitting distribution was selected based on the fit of the curve during the trial period [visual fit—the curves were compared to the KM curves; statistical fit—informed by Akaike information criterion (AIC) and the appropriateness of the extrapolation beyond the trial period (expert opinion)] [18].
Patients were modeled to start in the pre-progression health state and receive second-line hormonal therapy (i.e., fulvestrant 500 mg, anastrozole, letrozole, or exemestane) until disease progression. After disease progression while receiving second-line hormonal therapy, patients move to the post-progression health state, receive subsequent therapies, and remain in this state until death. Patients can also transition to the death state during each cycle, based on estimates of OS (Fig. 1).
A cycle length is 1 month, which resembles the treatment and follow-up scheme in the CONFIRM trial. The economic evaluation was performed from a Swedish national payer perspective. No indirect and direct non-medical costs were included in the analysis. Both the future costs and benefits were discounted by 3% over the duration of the model time horizon [19]. Half-cycle correction was applied to all outcomes except for adverse events which were assumed to occur as one-off events. The model was validated internally and externally [19]. The OS/PFS survival curves were compared against CONFIRM clinical trial estimates; values generated by the model and overall validation of the model structure and applicability to the disease area were assessed by clinical experts. The model was deemed to be appropriate for the decision problem in regards to the model structure and inputs.
2.3 Model Inputs
2.3.1 Health Effects
2.3.1.1 Survival Distributions for Overall Survival and Progression-Free Survival
Parametric distributions recommended by National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) were fit to the CONFIRM patient-level data [20]. For OS, the Weibull distribution was chosen as the best-fitting distribution according to the visual/statistical fit of the curve (Electronic Supplementary Material) and appropriateness for extrapolation beyond the trial, which was validated by clinical experts [21]. The robustness of the incremental cost-effectiveness ratio (ICER) was tested in sensitivity analysis by fitting the gamma (showed good fit to the CONFIRM data but was rejected as clinically implausible) and the exponential (showed similar fit to Weibull) distributions.
Within the CONFIRM trial, time to progression (TTP) included disease progression or death, which is more commonly referred to as PFS; hence, we refer to PFS herein. Based on statistical fit, the log-normal distribution provided the best fit for PFS in the CONFIRM trial. Yet, as indicated by a cumulative hazard plot, it showed a steadily increasing overestimation of PFS over time (Electronic Supplementary Material). Consequently, a two-part model splitting PFS into two phases, up to 180 days and 180+ days, was used. This timepoint was informed by protocol assessment of patient progression and the time the patients needed to achieve full treatment effectiveness. To better reflect the observed treatment efficacy in the clinical trial advised by the NICE evidence review group, the initial 180 days were modelled using the KM data for fulvestrant 500 mg from the CONFIRM study, which informed the baseline survivor function. For 180+ days, the exponential function was chosen for extrapolation given an observed linear trend of cumulative PFS hazard from 6 months onwards (Electronic Supplementary Material) [22]. The exponential distribution applied for the whole study period and the log-normal distribution providing the best statistical fit to patient-level data was tested in sensitivity analysis.
2.3.1.2 Comparative Effectiveness
There were no head-to-head randomized clinical trials comparing fulvestrant 500 mg to AIs as a second-line therapy in an ER+, postmenopausal ABC population. Direct evidence was only available for the fulvestrant 500 mg dose versus the 250 mg dose [14, 15]. Hence, a network meta-analysis (NMA) allowing comparison between fulvestrant 500 mg and AIs was needed.
A systemic review was performed which identified ten unique studies [10, 11, 14, 23,24,25,26,27,28,29,30] relevant for the NMA. Since OS and PFS data were available from multiple trials across the comparators, it was necessary to pool the available data. Networks were created for OS, PFS, and serious adverse events (SAEs) (Electronic Supplementary Material). Based on a network of clinical trials, the NMA approach was applied to indirectly estimate the relative efficacy (HRs) of fulvestrant 500 mg versus AIs in the targeted population. Treatments included in the network were comparators of interest: anastrozole 1 mg, exemestane 25 mg, letrozole 2.5 mg, and fulvestrant 250 mg, which served as a link between fulvestrant 500 mg and other ETs. Megestrol acetate 40 mg and a fulvestrant 250 mg loading dose were only used as connectors. When modelling the study-level data using parametric survival distributions, the proportionality assumption was valid and the difference between treatment arms could be summarized by a single number: the HR. As a result, it was possible to perform a NMA by pooling the HRs for PFS and OS across the interventions and extrapolating the OS/PFS by using selected distributions.
Data were analyzed using a fixed-effect NMA and a Bayesian approach. The model parameters were estimated using Markov chain Monte-Carlo techniques with WinBUGS (MRC Biostatistics Unit, Cambridge, UK). HRs for the comparators versus fulvestrant 500 mg were derived for both PFS and OS. The NMA showed that HRs favored fulvestrant 500 mg over all other comparators for OS and PFS, both in the 0- to 180-day analysis and in the analysis of 180+ days. The HRs were applied to the survival curves for fulvestrant 500 mg to derive extrapolated PFS/OS survival curves for each comparator in the model. Comparative effectiveness data are presented in Table 1. More detailed description of the methods applied in the NMA can be found in the study by Telford et al. [21], which evaluated the relative efficacy, in terms of OS, of fulvestrant versus other therapies in ABC. Yet, there is a marginal difference between the efficacy parameters used for this analysis and those published in the study by Telford et al. [21] as it included comparators that were beyond the scope of this analysis (everolimus + exemestane). Therefore, comparative efficacy for our analysis was sourced from a separate NMA [21] developed specifically for the Swedish setting.
2.3.2 Adverse Events
Only SAEs that lead to deterioration in quality of life and require healthcare services were incorporated into the model. SAEs data were sourced from the NMA and were analyzed as event rates whereby the data are expressed as total number of events per patient-year exposed (Table 1). No information on duration of SAEs was available; therefore, all SAEs were assumed to be handled over one hospitalization day. SAEs were incorporated into the economic model through applying the proportion of patients expected to experience a SAE per year for each treatment from the NMA.
2.3.3 Costs
The resource use and costs were based on both published sources and expert assessment. An oncologist at Stockholm South General Hospital was consulted regarding treatment patterns for ABC in Sweden. Unit costs for medical resources were taken from publicly available price lists in Sweden [31,32,33,34,35,36]. Medication acquisition costs along with resource use and associated costs are presented in Table 2. All costs used were converted to euros [1 Swedish kronor (SEK) = €0.10678] and valued for 2015/2016.
2.3.3.1 Pre-Progression State
Healthcare resource utilization associated with second-line hormonal therapy included hormonal therapy costs, treatment-related resource use for drug administration, treatment-independent resource use for routine care (such as monitoring disease progression), and resource use associated with SAEs associated with treatment. Medication acquisition costs and their sources are presented in Table 2. Treatment-related and independent resource use associated with monitoring disease progression was based on expert opinion and is presented in Table 3. An initial oncology visit for treatment initiation was assumed for all hormonal therapies. For fulvestrant 500 mg, one outpatient oncology nurse visit for drug injection every month with additional injection in the first month was assumed. To handle SAEs associated with hormonal therapies, 1 day of hospital admission (€888) was assumed.
2.3.3.2 Post-Progression State
Following progression, patients enter the post-progression health state where they receive a sequence of treatments, including third-line hormonal therapy, chemotherapy, and supportive palliative care. To estimate the post-progression health state cost, it was assumed that all patients entering the post-progression health state receive the same treatment options, irrespective of their previous treatment as there are no data from clinical trials or observational studies in Sweden to indicate the medication that is most commonly received as third-line hormonal therapy. Therefore, the cost of third-line hormonal therapy was based on an average cost of hormonal therapies consisting of fulvestrant 500 mg, anastrozole, letrozole, exemestane, tamoxifen, or everolimus + exemestane and respective treatment-related costs associated with therapy administration. The choice of third-line ET was based on Swedish guidelines and expert opinion. The post-progression health state per patient costs consist of a one-off treatment cost (€19,406), including third-line hormonal therapy (€3408; Table 2), chemotherapy (€8649; Table 2), and supportive palliative care (€7349; Table 3). Relative dose intensity was not considered. Monthly disease monitoring costs (€455; Table 3) were applied for each cycle until death.
2.3.4 Health-Related Quality of Life
Utilities reflect the preference for a certain health state and are measured on a 0–1 scale (1 reflects perfect health and 0 represents death) [17]. Utility values for health states in the model were acquired from a study by Lloyd et al. [37], which was identified from a systematic review. The utilities reported by Lloyd et al. [37] were elicited from the general UK public using a standard gamble method. Utilities were estimated for distinct states of metastatic BC, yet were not specific to any cancer treatment. To better reflect quality of life experienced when receiving hormonal therapy, the utility for the pre-progression state was based on the utility for stable cancer patients on treatment without toxicity. To better reflect the targeted population, utility values were adjusted based on an average age (56 years) of Swedish metastatic BC patients [38]. The utility value of 0.7938 was applied to the ‘pre-progression’ state and 0.5498 for the ‘post-progression’ state. Disutilities due to SAEs were omitted from the model as the differences in adverse events rates between treatments were not considered significant enough to influence differences in quality of life. However, as the SAE rate for fulvestrant 500 mg was the second lowest, this assumption favors the comparators.
2.3.5 Sensitivity Analysis
In order to identify the top 5 drivers of the results, one-way sensitivity analyses varying parameters by their high and low values were performed. High and low values for efficacy parameters were sourced from NMA [95% credible intervals (CrIs)], for utilities and costs ±10% change was assumed and the discount rate for both costs and discount rates of 0 and 5% were used in sensitivity analysis [19]. Alternative distributions for extrapolation of OS and PFS were tested. Probabilistic sensitivity analysis (PSA) was conducted to assess the parametric uncertainty associated with the base-case results. Parameter uncertainty was assessed by assigning probability distributions [PFS and OS for baseline curve—multivariate normal distribution; HRs—sampled directly from WinBUGS CODA (Convergence Diagnostic and Output Analysis); proportion of patients with SAE, proportion of patients being monitored each month, and utilities—β distribution; hospital length of stay with SAEs—lognormal distribution] and point estimates were drawn using Monte-Carlo simulation techniques (10,000 iterations). The known correlation between parameters were preserved where possible. The correlations for baseline survival curve parameters (PFS and OS) were available from the survival analysis and were included in the model (assuming a multivariate normal distribution). For HRs, the parameter estimates were preserved by sampling values from the same Markov chain Monte-Carlo iteration.
3 Results
Over a lifetime, treatment with fulvestrant 500 mg was associated with a higher total cost and greater health gains in terms of QALYs and LYs gained compared to AIs. The incremental cost per QALY gained was €33,808 (incremental cost of €13,283; incremental QALY of 0.393), €33,883 (incremental cost of €14,986; incremental QALY of 0.442), and €49,225 (incremental cost of €13,862, incremental QALY of 0.282) versus anastrozole, letrozole, and exemestane, respectively. The incremental cost per LY gained was €21,312 (incremental LY of 0.623), €20,338 (incremental LY of 0.737), and €27,854 (incremental LY of 0.498) versus anastrozole, letrozole, and exemestane, respectively (Table 4). Incremental cost-effectiveness results when ranking in ascending order of total costs are presented in Table 4. No treatment was strictly dominated.
In the PSA, fulvestrant 500 mg was associated with an ICER of €35,517, €35,892, and €51,574 in comparison with anastrozole, letrozole, and exemestane, respectively (Table 4). For cost-effectiveness thresholds of €50,000, €80,000, or €100,000 (cost/QALY), the probability of fulvestrant being cost effective is 46, 65, and 70%, respectively. For the respective willingness-to-pay (WTP) thresholds, the probability of being cost effective is 4, 2, and 2% for anastrozole, 13, 9, and 8% for letrozole, and 37, 23, and 20% for exemestane (Fig. 2).
The results were largely driven by HRs for PFS and OS sourced from the NMA (Table 5). When varying HRs for PFS and OS to their CrIs from NMA, ICERs ranged from fulvestrant being dominated (fulvestrant was associated with fewer QALYs and greater costs than AIs) to €20,980. Other than HRs for OS and PFS, the cost-effectiveness results were most sensitive to varying discount rates and utilities, although the variability was generally small (Table 5). Other parameters did not have a large effect on the results. When scenarios assuming alternative distributions for extrapolating OS (gamma and exponential parametric function) and PFS (exponential and log-normal for the whole time period) were tested, the results remained stable for PFS but showed a small increase for OS.
4 Discussion
Fulvestrant 500 mg is a well-documented drug in terms of efficacy, tolerability, and safety in patients with ABC. Fulvestrant 500 mg is included in the international and Swedish treatment guidelines among the recommended ETs in metastatic BC as a second- and later-line treatment [6, 7, 39, 40].
There is no official threshold for the WTP per QALY gained in Sweden. However, information regarding the threshold for outpatient prescription pharmaceuticals can be inferred from previous reimbursement decisions in Sweden, with an implied WTP for a QALY between €80,000 and €135,000 depending on disease severity [41]. Yet, €100,000 is a commonly used WTP threshold for oncology products in Sweden and as a value beyond which the likelihood of a product being reimbursed substantially decreases [41]. The model-based economic analysis showed that at a WTP threshold of €100,000 for fulvestrant 500 mg appears to be a cost-effective alternative to anastrozole, letrozole, and exemestane with an incremental cost per additional QALY of €33,808, €33,883, and €49,225, respectively. Within sensitivity analysis, applying the upper and lower CrIs for PFS and OS had the greatest effect on the ICER. Other parameters did not have a large effect on the results.
The results of our study are in line with earlier published results. A previous economic evaluation of fulvestrant 500 mg versus generic anastrozole and letrozole was conducted in the UK [42] with ICERs of £31,468 and £34,528, respectively. The study applied a very similar approach by sourcing PFS/OS for fulvestrant from the CONFIRM trial and comparative effectiveness from an NMA [43]. However, the economic evaluation reported here was based on an NMA that used a more mature OS dataset from CONFIRM [14] and employed a two-part model approach for PFS, which is in line with recommendations from the NICE evidence review group. Our study also included exemestane. The economic evaluation conducted here also used a different approach for post-progression costs estimation, which reflects Swedish clinical practice better. The post-progression costs were estimated by applying a treatment-skipping approach assuming that not all patients experience the same treatment sequence, which was the case in our analysis where an average cost across different regimens was calculated. Our analysis applied a different hormonal and chemotherapy scheme. There were differences in the way post-progression costs were applied. In our study, costs were applied as a lump sum once patients entered the post-progression state, whereas monthly costs were applied as long as a patient stayed alive in the Das et al. [42] study. Given that fulvestrant was associated with longer life expectancy, the latter approach led to higher costs in the fulvestrant arm and favored comparators. Further, fulvestrant 500 mg has been accepted for use in NHS Scotland, with ICERs of £20,859, £19,981, and £24,539 versus anastrozole, letrozole, and exemestane, respectively [44].
Fulvestrant was found to be a cost-effective treatment alternative in ER+ ABC at a lower dose of 250 mg. The cost effectiveness of a second-line treatment sequence with and without fulvestrant 250 mg was assessed in Germany [45] and the UK [46]. Both studies concluded that fulvestrant 250 mg is a valuable ET in ABC, leading to cost savings in the German study and an ICER of £7500/QALY in the UK study.
Not many studies have previously been published regarding the cost effectiveness of pharmacological interventions against BC in Sweden. Lundkvist et al. [47] evaluated the cost effectiveness of exemestane versus tamoxifen as adjuvant therapy for early-stage BC after 2–3 years’ treatment with tamoxifen (ICER of €20,000). Lidgren et al. studied the cost effectiveness of HER2 testing and both 1-year adjuvant trastuzumab therapy for early BC (ICER of €36,000–41,500) [48] and metastatic cancer (ICER of €52,300–60,400) [49].
The uncertainty associated with efficacy data sourced from the NMA is the major limitation of the study as varying HRs for PFS and OS led to relatively big changes in results according to their 95% CrIs in the NMA. HRs sourced from the NMA are more uncertain than those from head-to-head studies as additional assumptions and advanced statistical analysis are required. Factors such as connecting fulvestrant 500 mg to comparators through fulvestrant 250 mg, limited number of clinical trials, and a small trial sample size contributed to higher uncertainty and wider CrIs for PFS/OS. Varying HRs for PFS/OS according to their 95% CrIs from the NMA resulted in clinically implausible scenarios, particularly when using the lower CrIs, where patients survived for much longer than expected. Hence, sensitivity analysis results should be interpreted with caution. Sourcing relative efficacy data from the unpublished NMA is another limitation of our study, yet it is closely related to the published NMA.
Although there are no direct comparative studies with AIs in the second-line indication, studies assessing the efficacy of fulvestrant 500 mg versus anastrozole in other therapy lines support the results of indirect comparison used for this analysis. Fulvestrant 500 mg demonstrated significantly improved TTP versus anastrozole 1 mg [23.4 vs. 13.1 months; HR = 0.66 (95% CI 0.47–0.92)] in a phase II [FIRST (Fulvestrant fIRst-line Study comparing endocrine Treatments)] trial in a first-line setting [50]. These findings are tested in a phase III [FALCON (Fulvestrant 500 mg Versus Anastrazole 1 mg for Hormone Receptor-Positive Advanced Breast Cancer), NCT01602380] trial for first-line hormone-naïve patients, which has recently met the primary endpoint [16.6 vs. 13.8 months; HR = 0.797 (95% CI 0.637–0.999); p = 0.0486] [51].
Given the highly individualized treatment of ABC, alternative treatments are needed. Yet, budgetary limitations and treatments varying in efficacy and price make decision making difficult. Therefore, a comprehensive approach assessing additional health benefits in the light of additional costs to improve value to society should be used to facilitate decision making [52].
5 Conclusions
Based on a recent NMA combined with health economic modelling, fulvestrant 500 mg brings additional health gains at additional costs compared to anastrozole, letrozole, and exemestane. At a WTP of €100,000/QALY, fulvestrant 500 mg may be a cost-effective option compared to AIs in Swedish postmenopausal women with ER+, locally advanced, or metastatic BC who relapse during or after previous ET.
References
Socialsyrelsen. Cancer incidence in Sweden. Stockholm, Sweden: Sveriges Officiella Statistik: Statistik—Hälso- och Sjukvård; 2011.
Regionala Cancercentrum i Samverkan. Bröstcancer: Nationellt vårdprogram: Landstingens och regionernas nationella samverkansgrupp inom cancervården. Sweden: Regionalla cancercentrum i samverkan; 2014.
Socialstyrelsen. Öppna jämförelser cancersjukvård: jämförelser mellan landsting. Stockholm, Sweden: Socialstyrelsen; 2014.
Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–52. doi:10.1016/j.breast.2012.03.003.
Ciruelos E, Pascual T, Arroyo Vozmediano ML, Blanco M, Manso L, Parrilla L, et al. The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. Breast. 2014;23(3):201–8. doi:10.1016/j.breast.2014.01.016.
Socialstyrelsen. Nationella riktlinjer för bröst-, prostata-,tjocktarms- och ändtarmscancervård. Stockholm, Sweden: Socialstyrelsen; 2015.
NCCN. Clinical practice guidelines in oncology: breast cancer. Fort Washington: National Comprehensive Cancer Network; 2015.
Di Leo A, Curigliano G, Dieras V, Malorni L, Sotiriou C, Swanton C, et al. New approaches for improving outcomes in breast cancer in Europe. Breast. 2015;24(4):321–30. doi:10.1016/j.breast.2015.03.001.
Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer. 2006;42(17):2867–75. doi:10.1016/j.ejca.2006.08.010.
Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–95.
Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–403.
Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22(9):1605–13. doi:10.1200/jco.2004.02.112.
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–70. doi:10.1200/jco.2007.13.5822.
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Final overall survival: fulvestrant 500 vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337. doi:10.1093/jnci/djt337.
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–600. doi:10.1200/jco.2010.28.8415.
European Medicines Agency. Assessment report for Faslodex (fulvestrant). 2010 Contract No.: EMEA/H/C/000540/II/0018. London: European Medicines Agency; 2010.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi:10.1093/bmb/ldq033.
Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2002.
The Dental and Pharmaceutical Benefits Agency. General recommendations for economic evaluations. Stockholm: The Dental and Pharmaceutical Benefits Agency; 2003.
Latimer N. NICE DSU Technical Support Document 14: survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data. Sheffield: Decision Support Unit; 2011.
Telford C, Jones N, Livings C, Batson S. Network meta-analysis comparing overall survival for fulvestrant 500 mg versus alternative therapies for treatment of postmenopausal, estrogen receptor-positive advanced breast cancer following failure on prior endocrine therapy. Clin Breast Cancer. 2016;16(3):188–95. doi:10.1016/j.clbc.2016.02.007.
National Institute for Health and Care Excellence. Fulvestrant for the treatment of locally advanced or metastatic breast cancer. Technology appraisal guidance [TA239]. London: NICE; 2011.
Buzdar AU, Jonat W, Howell A, Jones SE, Blomqvist CP, Vogel CL, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Arimidex Study Group. Cancer. 1998;83(6):1142–52.
Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19(14):3357–66.
Ohno S, Rai Y, Iwata H, Yamamoto N, Yoshida M, Iwase H, et al. Three dose regimens of fulvestrant in postmenopausal Japanese women with advanced breast cancer: results from a double-blind, phase II comparative study (FINDER1). Ann Oncol. 2010;21(12):2342–7. doi:10.1093/annonc/mdq249.
Pritchard KI, Rolski J, Papai Z, Mauriac L, Cardoso F, Chang J, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res Treat. 2010;123(2):453–61. doi:10.1007/s10549-010-1022-9.
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–98. doi:10.1016/s1470-2045(13)70322-x.
Xu B, Jiang Z, Shao Z, Wang J, Feng J, Song S, et al. Fulvestrant 250 mg versus anastrozole for Chinese patients with advanced breast cancer: results of a multicentre, double-blind, randomised phase III trial. Cancer Chemother Pharmacol. 2011;67(1):223–30. doi:10.1007/s00280-010-1483-x.
Jiang Z. A phase III study of fulvestrant 500 mg versus 250 mg in postmenopausal Chinese women with advanced breast cancer and disease progression following failure on prior antiestrogen or aromatase inhibitor therapy [abstract no. P1-13-07]. Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; 9–13 Dec 2014; San Antonio; 2014.
Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–84. doi:10.1007/s12325-013-0060-1.
Price list for Region Södra. 2015. https://www.skane.se/sv/Webbplatser/Sodra-regionvardsnamnden/Regionala-priser-och-ersattningar-for-Sodra-sjukvardsregionen/. Accessed March 2017.
Price list for Norrlandstingen Region. 2016. http://www.norrlandstingen.se/halso-och-sjukvard/avtal-och-priser/prislistor-norra-sjukvardsregionen/. Accessed March 2017.
Price list for Stockholm and Gotland Region. 2016. http://66.7.199.160/~sthlmgot/2016-2/. Accessed March 2017.
Price list for Sydöstra Region. 2016. http://plus.rjl.se/infopage.jsf?nodeId=41089. Accessed March 2017.
Price list for Västra Götaland Region. 2016. http://www.vgregion.se/sv/Vastra-Gotalandsregionen/startsida/Politik/Namnder-och-styrelser/Namnder-och-styrelser-for-halso–och-sjukvard/Samverkansnamnden/. Accessed March 2017.
Price list for Uppsala Örebro Region. 2016. http://svnuppsalaorebro.se/3-styrande/prislista.html. Accessed March 2017.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90. doi:10.1038/sj.bjc.6603326.
Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–81. doi:10.1007/s11136-007-9202-8.
Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–69. doi:10.1200/jco.2013.54.2258.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489–502. doi:10.1016/j.breast.2014.08.009.
Svensson M, Nilsson FO, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33(11):1229–36. doi:10.1007/s40273-015-0307-6.
Das R, Cope S, Ouwens M, Turner P, Howlett M. Economic evaluation of fulvestrant 500 mg versus generic nonsteroidal aromatase inhibitors in patients with advanced breast cancer in the United Kingdom. Clin Ther. 2013;35(3):246–60. doi:10.1016/j.clinthera.2013.01.011.
Cope S, Ouwens MJ, Jansen JP, Schmid P. Progression-free survival with fulvestrant 500 mg and alternative endocrine therapies as second-line treatment for advanced breast cancer: a network meta-analysis with parametric survival models. Value Health. 2013;16(2):403–17. doi:10.1016/j.jval.2012.10.019.
Advice for fulvestrant (Faslodex) [database on the Internet] 2016. https://www.scottishmedicines.org.uk/SMC_Advice/Advice/114_04_fulvestrant_Faslodex/fulvestrant_Faslodex_Resubmission. Accessed Apr 2017.
Lux MP, Hartmann M, Jackisch C, Raab G, Schneeweiss A, Possinger K, et al. Cost-utility analysis for advanced breast cancer therapy in Germany: results of the fulvestrant sequencing model. Breast Cancer Res Treat. 2009;117(2):305–17. doi:10.1007/s10549-008-0294-9.
Cameron DA, Camidge DR, Oyee J, Hirsch M. Economic evaluation of fulvestrant as an extra step in the treatment sequence for ER-positive advanced breast cancer. Br J Cancer. 2008;99(12):1984–90. doi:10.1038/sj.bjc.6604790.
Lundkvist J, Wilking N, Holmberg S, Jonsson L. Cost-effectiveness of exemestane versus tamoxifen as adjuvant therapy for early-stage breast cancer after 2–3 years treatment with tamoxifen in Sweden. Breast Cancer Res Treat. 2007;102(3):289–99. doi:10.1007/s10549-006-9333-6.
Lidgren M, Jonsson B, Rehnberg C, Willking N, Bergh J. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol. 2008;19(3):487–95. doi:10.1093/annonc/mdm488.
Lidgren M, Wilking N, Jonsson B, Rehnberg C. Cost-effectiveness of HER2 testing and trastuzumab therapy for metastatic breast cancer. Acta Oncol. 2008;47(6):1018–28. doi:10.1080/02841860801901618.
Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–11. doi:10.1007/s10549-012-2192-4.
Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. doi:10.1016/S0140-6736(16)32389-3.
Salas-Vega S, Mossialos E. Cancer drugs provide positive value in nine countries, but the United States lags in health gains per dollar spent. Health Aff (Millwood). 2016;35(5):813–23. doi:10.1377/hlthaff.2015.1453.
Author information
Authors and Affiliations
Contributions
US, ME, DT, CT, and CL have made substantial contributions to the work reported in the manuscript and take public responsibility for part (DT, CT, CL) or the entire content (US, ME) of the manuscript. DT provided medical information, clinical trial data, and contributed to the overall text of the manuscript; CT and CL provided data for comparative efficacy from the NMA, contributed to economic model development, and contributed to the overall text of the manuscript; ME and US performed country adaptations to the economic model, input data collection, and analysis, and contributed to the overall text of the manuscript.
Corresponding author
Ethics declarations
Funding
The study was funded by AstraZeneca Nordic-Baltic. Authors Ugne Sabale, Mattias Ekman, Daniel Thunström, Claire Telford, and Christopher Livings are employees at AstraZeneca.
Data availability
The data that support the findings of this study are available from AstraZeneca but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of AstraZeneca and the clinical trial investigators.
Additional information
The work was performed while at DRG Abacus relates to Christopher Livings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sabale, U., Ekman, M., Thunström, D. et al. Economic Evaluation of Fulvestrant 500 mg Compared to Generic Aromatase Inhibitors in Patients with Advanced Breast Cancer in Sweden. PharmacoEconomics Open 1, 279–290 (2017). https://doi.org/10.1007/s41669-017-0031-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-017-0031-6