Abstract
With the consumption of energy and the spread of COVID-19, the demand for ethanol production is increasing in the world. The industrial ethanol fermentation microbes cannot metabolize the alginate component of macro algae, which affects the ethanol yield. In this research, the ethanol production process from macro algae by an alginate fermentation yeast Meyerozyma guilliermondii, especially the pretreatment process of Colpomenia sinuosa, was studied. At the same time, the experimental design of Box-Behnken was carried out to achieve the optimum fermentation performance. The concentration of KH2PO4 (A: 2–6 g.L−1), pH (B: 4–7), reaction time (C: 60–120 h) and temperature (D: 24–34 °C) were variable input parameters. During the ethanol production process, the algae powder was firstly mixed with water at 90 °C for 0.5 h. Later the fermentation culture medium was prepared and then it was fermented by the yeast Meyerozyma guilliermondii to produce ethanol. And the optimal fermentation parameters were as follows: fermentation temperature of 28 °C, KH2PO4 dosage of 4.7 g.L−1, initial pH of 6, and fermentation time of 99 h. The ethanol yield reached 0.268 g.g−1 (ethanol to algae), close to the predicted value of model. The generation of alginate lyase during the fermentation of algae was also examined. The highest alginate lyase activity reached 46.42 U.mL−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioethanol is one of the most representative energy alternatives in the global to alleviate the shortage of fossil oil resources [1]. The use of bioethanol can also effectively reduce the emissions of nitrogen oxides, hydrocarbons and carbon monoxide [2]. At the same time, during the epidemic period of COVID-19, bioethanol as an effective disinfectant also has a great demand [3]. However, the production of bioethanol encountered various problems. The large-scale production of ethanol from corn and sugarcane may affect the global food security [4]. Cellulosic ethanol can take straw, bagasse and sawdust as raw materials [5, 6], but low ethanol yield and high economic and environmental costs limit its large-scale development [7, 8]. The content of carbohydrate in the macro-algae is rich (more than 50%) [9]. Compared with other raw materials, the macro-algae have obvious advantages in ethanol production [10, 11]. Moreover, algae do not compete with terrestrial plants for arable land, water, fertilizer and/or other production resources. Algae can also fix carbon dioxide in the atmosphere and sea water, and effectively alleviate the greenhouse effect and acidification of sea water [12]. Therefore, macro-algae are considered as a kind of energy biomass with broad prospects.
However, the bioconversion from macro-algae to ethanol also has some difficulties. Alginate, the main polysaccharide component in macro-algae, is not easy to be utilized by traditional ethanol fermentation microorganisms [13]. Alginate often exists in the form of alginate (salt) because it contains carboxyl group. There are two main solutions to this problem. The first method is to use traditional ethanol fermentation microorganisms [14], which does not need to change the original fermentation process, but alginate cannot be utilized. Therefore, the ethanol yield is relatively low [15], and the residue would cause environmental problems. The second method is to directly construct genetic engineering microorganisms to realize the transformation from alginate to ethanol. Researchers have studied many methods, including constructing ethanol metabolism pathway in alginate degrading bacteria [16], combining alginate lyase gene with Saccharomyces cerevisiae genome to realize the effective degradation of sodium alginate by yeast [17], and completely constructing the metabolism pathway from alginate to ethanol in Escherichia coli [15] to solve the problem of ethanol transformation of alginate. But because the constructed genetic engineering strains are mostly aerobic microorganisms, the growth and ethanol metabolism process would inhibit each other. Meanwhile, the oxygen content in the reactor must be strictly controlled, which limits the practical application. The genetic stability and biological safety of the genetic engineering microorganisms also need to be further tested. Based on the problems of the above two methods, researchers isolated strains that can directly use alginate to produce ethanol in nature. Ji et al. [18] screened the strain Defluviitalea phaphyphila that can ferment glucose, mannitol and alginate to produce ethanol. However, this strain belongs to the thermophilic bacteria, and the utilization rate of glucose and alginate at 60 °C was much higher than 45 °C. High fermentation temperature is not conducive to industrial application.
Considering all factors, the appropriate solution should not only realize the effective utilization of alginate, but also be stable and easy to operate in industrial production. In our previous study, several strains with ability to produce ethanol from alginate were selected after isolation, TTC selection and fermentation experiments. And the ethanol yield of strain 5 was the highest and it was 99% identical to Meyerozyma guilliermondii (the Accession No. was EF375700). Meyerozyma guilliermondii can produce alcohol dehydrogenase (ADH) and alginate lyase to realize the alginate fermentation [19]. It can also utilize other sugars such as mannitol and glucose. And the anaerobic and non-thermophilic fermentation of this yeast does not need to change the original ethanol fermentation process. The ethanol fermentation from algae by Meyerozyma guilliermondii has a good application prospect. After optimization and treatment, the ethanol yield can also be improved.
Colpomenia sinuosa is a kind of typical brown algae. It is widely distributed in the coastal waters of China. Most of the Colpomenia sinuosa is not eaten directly as food, but often used as the raw material to produce alginate and seaweed extract fertilizer [20, 21]. The alginate content of Colpomenia sinuosa is relatively high compared to Undaria pinnatifida and Scagassum pallidum, so it is very suitable for ethanol fermentation by the natural alginate fermentation strain proposed in this study. At present, there is no research report on the production of ethanol from Colpomenia by alginate fermentation strain. In this research, Meyerozyma guilliermondii was used for the ethanol production from Colpomenia sinuosa, and the ethanol production process were also studied. At the same time, to reach the optimal fermentation performance, the pretreatment process and the Box-Behnken experimental design of ethanol fermentation were carried out. The most important parameters, which affect the production ability, were analyzed. The optimal fermentation parameters were also determined.
Materials and Methods
Materials
All analytical reagent grade chemicals were sourced from Chemical Industry Co., Ltd (Beijing, China). All biochemical reagents were purchased from Bio-Technology Factory (Beijing, China). The alginate fermentation yeast Meyerozyma guilliermondii was obtained from our previous study [19]. The yeast was stored at 4 °C. Colpomenia sinuosa (Alginate content:25.1 ± 1.2%) was obtained from a market in Hangzhou, China. The samples were first washed by clean water for three times. Then the samples were dried in the incubator at 60 °C overnight. Dry algae were finely grinded down with a ball miller and then stored at 4 °C.
Culture Media and Microorganism Culture
YPD medium was used as the enrichment medium containing 10 g.L−1 yeast extract, 20 g.L−1 peptone and 20 g.L−1 glucose. 2% agar was added to the YPD medium to make YPD solid medium. The composition of adaptation culture medium was: 2% peptone, 1% yeast extract, and 2% alginate. The composition of fermentation culture medium was: 10.8 g.L−1 (NH4)2SO4, 5.0 g.L−1 KH2PO4, 1.1 g.L−1 MgSO4·7H2O, and algae solution after pretreatment. During the Box-Behnken experiment, the concentration of KH2PO4 was varying from 2 to 6 g.L−1.
The Pretreatment of Macro-Algae
To improve the utilization rate of macro-algae, the algae were treated by different pretreatment methods including heating and enzymatic hydrolysis.
Heating Pretreatment with Water as Solvent
Different amount of algae powder was mixed with water (the concentration of algae was from 10 g.L−1 to 40 g.L−1), and then the solution was heated to 90 °C for 0.5 h. The treatment temperature and time were determined according to our previous experiments. Such treatment was conducive to the production of soluble sugar in the solution.
Enzymatic Hydrolysis Pretreatment
Firstly, the algae powder was dissolved in citric acid-sodium citrate buffer (the concentration of algae was from 10 g.L−1 to 40 g.L−1). Then the samples were supplemented with 10.0 FPU.g−1 DM cellulase (Accellerase 1000, 55.0 FPU.mL−1) and kept at 40 °C for 3 h according to the reference [22]. In the process of enzymatic hydrolysis, sodium hydroxide solution was used to adjust the pH value of the solution to 5.
The Ethanol Fermentation Process
Adaptation Culture of Yeast
The stored yeast was firstly transferred to YPD solid medium and cultured at 28 °C for 48 h. Then, the activated yeast was transferred to the adaptation culture medium and cultured at 28 °C for 48 h. Then the yeast was transferred to the medium again for the second culture with 10% inoculum.
Preparation of Fermentation Medium
The nutrient was added to the pretreated seaweed solution to prepare the fermentation medium. Then the fermentation medium was sterilized at 115 °C for 20 min. After sterilization, the medium was cooled to room temperature.
Batch Fermentation
The yeast was inoculated into the fermentation medium with 10% inoculum. The flasks with fermentation medium were capped with butyl rubber covers to create an anaerobic condition. All the flasks were cultured in a shaking incubator, with the speed of 150 r.min−1. The blank test was set in the same operation mode without adding microorganisms or any other solution. The results showed that ethanol was not produced in the blank test.
Box-Behnken Experimental Design
The experimental parameters and their range for Box-Behnken experimental design were determined according to the single factor experiments in our previous study. Through the single factor experiments, the parameter range with higher yield was obtained. The value of the ethanol concentration was measured under different concentration of KH2PO4, temperature, fermentation time and pH value. Experimental design of the box Behnken fermentation process was carried out by Design Expert 12 software. The concentration of KH2PO4 (A: 2–6 g.L−1), pH (B: 4–7), reaction time (C: 60–120 h) and temperature (D: 24–34 °C) were variable input parameters. The experimental data was subjected to quadratic polynomial fitting using the nonlinear regression method to determine the relevant model terms. Considering all the linear terms, square terms and linear by linear interaction items, the quadratic response model can be described as:
In which, βo is the offset term, βi is the slope or linear effect of the input factor xi, βii is the quadratic effect of input factor xi and βij is the linear by linear interaction effect between the input factor xi and xj.
Analytical Methods
The content of soluble sugar and reducing sugar was determined by phenol–sulfuric acid method and DNS method respectively [23, 24]. The pH was measured in a pH meter (PHS-3B, Shanghai Precision& Scientific Instrument Co. Ltd, China). The optical density (OD) of cell growth at regular intervals was determined at 600 nm using a UV–VIS Spectrophotometer (TU-1901, Purkinje General Instrument Co. Ltd, China) throughout the study. The fermentation broth was distilled first and then ethanol concentration was determined by sulfuric acid- potassium bichromate method [25]. The mass of ethanol produced was the volume of distillate (L) multiplied by the ethanol concentration (g L−1). The enzyme activity of alginate lyase was measured according to the reference [19]. The components of Colpomenia sinuosa was measured by the following methods according to the reference [20]. The content of crude protein was determined by Kjeldahl method. The content of crude fiber was measured by weight method. The content of alginate was determined by calcium acetate method. Alginate content was calculated by alginic acid. The content of mannitol was determined by sodium thiosulfate titration. All experiments were carried out for three times.
The ethanol yield in the ethanol fermentation experiments was calculated as:
In which Sr was the initial mass of algae (g), se was the mass of ethanol (g) produced by fermentation.
Results and Discussion
Different Pretreatment Process
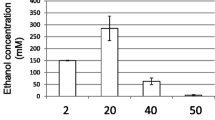
To improve the utilization rate of macro-algae, the Colpomenia sinuosa was treated by different pretreatment methods according to the “The Pretreatment of Macro-Algae” section. The best pretreatment method was determined by comparing the ethanol yield under different treatment conditions. The fermentation condition was as follows: initial pH of 7.0, fermentation temperature of 32 °C, inoculum amount of 10% and fermentation time of 72 h. The result was shown in Table 1.
According to the result, the substrate (algae) concentration had a great influence on ethanol yield. The ethanol yield of all the pretreatment methods was highest when the substrate concentration was 20 g.L−1. Because some insolubility substances still existed in the solution after substrate pretreatment. If the concentration of the substrate was too high, the increase of SS in the fermentation broth affected the respiration of yeast. A high substrate concentration would cause high viscosity in the broth, thus leading to the high transfer resistance and the heterogeneity of cell metabolism [26]. If the substrate concentration was decreased, the concentration of soluble sugar released in the solution was low, which may also affect the cell growth and ethanol yield. At the same time, after pretreatment, most of alginate still existed in the insoluble matter. Taking 20 g.L−1 algae treatment group as an example (the best reaction group), after heat treatment, the yield of solid insoluble matter (weigh after drying) was 30.12%, while the content of alginate in insoluble matter was 70.04%. After cellulase hydrolysis, the yield of solid insoluble matter was 22.13%, while the content of alginate in insoluble matter was 90.45%.
In addition, after heating treatment and enzymatic hydrolysis treatment, the ethanol yield could be significantly improved. In the fermentation process, cells grow first, and then release extracellular enzyme to realize the utilization of alginate. Therefore, the soluble sugar generated by pretreatment is beneficial to the cell growth and can significantly improve the yield of ethanol [27]. This point can also be confirmed by the measurement of soluble sugar. After heating treatment and enzymatic hydrolysis, the sugar content in the solution was significantly increased. For example, the crude fiber content of the dry Colpomenia sinuosa was about 6.4%. Cellulase can hydrolyze the fiber components in the substrate, and the content of soluble sugar was significantly increased, which could help the growth and reproduction of yeast. And algae also can release sugar after heating treatment [1]. The ethanol yield of heating treatment was slightly higher than that of enzyme treatment. At the same time, the treatment time of enzymatic hydrolysis was longer, so the heating treatment was selected as the pretreatment method of substrate in the following research.
Box-Behnken Experimental Design of Ethanol Production by Colpomenia
From the results of pretreatment, the optimum condition was obtained. So the algae powder was mixed with water and then the solution (20 g.L−1) was treated at 90 °C for 0.5 h. Later the fermentation culture medium was prepared for ethanol production. Under different temperature, fermentation time, concentration of KH2PO4 and pH, the value of the ethanol concentration was measured. These four factors were the most important according to the result of signal factor experiments. And the Box-Behnken experimental design of fermentation process was carried out.
Using the statistical design experiment, the different parameter combination was experimented, and the integrated effect of these four factors was analyzed. The R (response) result of ethanol production was measured and the measured response was shown in Table 2. Experimental results showed that the Box Behnken experimental design model provided a second-order polynomial equation that reflected the relationship between ethanol yield (R) and four parameters (A: potassium ion concentration, B: pH, C: reaction time, D: reaction temperature). The final equation by the actual factors was as follows:
To test the hypotheses for model parameters, ANOVA is a statistical method of subdividing the overall variation of the set of data into component parts associated with a source of variation [28]. The ANOVA for second-order equation in this model was shown in Table 3.
According to the ANOVA’s rule, the Model F-value of 13.95 means the model is significant and reliable. There is only a 0.01% chance that a "Model F-Value" this large could happen because of noise. Values of "Prob > F" less than 0.0500 indicate model terms are significant. In this case A, D, AC, A2, B2, C2, D2 are significant model terms. Values greater than 0.1000 indicate the model terms are not significant. The "Lack of Fit F-value" of 5.35 shows Lack of Fit was not significant relative to the pure error. And there is a 6.01% chance that a "Lack of Fit F-value" this large could happen because of noise.
Effect of Various Parameters on Ethanol Yield
According to the Box-Behnken experimental design and the results of ethanol yield, the effect of different factors on ethanol yield is plotted in Fig. 1. For example, in Fig. 1A, the points mean the ethanol yield value in four same experimental conditions (concentration of KH2PO4: 4 g.L−1; pH: 5.5; fermentation time: 90 h; temperature: 29 °C). With the increase of KH2PO4 dose, the ethanol yield was rising. KH2PO4 can promote yeast growth and fermentation. But with excessive concentration, the fermentation can be inhibited. Higher concentration of inorganic salt would lead to the increasing osmotic pressure on microorganisms, which would affect their life activities and finally decrease the fermentation efficiency.
The pH value is also an important factor affecting the fermentation process of ethanol. Acid conditions are more suitable for yeast fermentation [27]. The activity of the microbial enzyme has a constant pH adaptation range. But the environment of peracids could reduce the microbial utilization efficiency of carbon source [19]. In this research, the ethanol yield was also significantly affected by the initial pH of the solution. During the fermentation, because of the produce of CO2 and organic acid, the pH of the solution would be reduced. The most suitable initial pH for yeast fermentation is about 5–6.
The optimum growth temperature of microorganisms is always 25–35 °C. High temperature or low temperature affects the activity of microbial synthetic metabolic enzymes. Studies on the effect of temperature on yeast fermentation have been reported [27, 29], but the optimum temperature is different. Because the fermentation reaction was always exothermic. In this research, the optimal fermentation temperature was 28–29 °C.
The fermentation time had great effect on the ethanol yield too. With the continuous consumption of nutrients and the production of metabolites, the growth and enzyme activity of yeast changed at different time. The ethanol yield increased first and then decreased with the increase of fermentation time.
The single-factor test described the relationship between a single factor and the ethanol yield. To study the performance of various factors on the fermentation effect, the interaction of multiple factors on the ethanol yield was also analyzed.
Effect of Potassium Ion Concentration (A) and Reaction Time (C)
To study the interaction effect of potassium ion concentration (A) and reaction time (C) on ethanol fermentation, experiments were carried out by varying concentration of KH2PO4 from 2 g.L−1 to 6 g.L−1 and under different fermentation time from 60 to 120 h. The result was shown in Fig. 2 through Box-Behnken experimental design. At the fixed C (for example: 60 h), the ethanol yield improved with an increase of A but when the concentration of A reached 4 g.L−1, the ethanol yield was decreased. At the same time when the C was prolonged (for example: 120 h), the ethanol yield increased with the improving KH2PO4 concentration (A) and then it remained almost constant. This result also showed the continuous demand of KH2PO4 during the yeast fermentation. In the fermentation process, KH2PO4 can be supplemented during mid-last fermentation period to improve the fermentation efficiency.
Effect of pH (B) and Reaction Temperature (D)
To study the effect of pH and reaction temperature on ethanol yield, experiments were carried out by varying pH from 4 to 7 and under different temperature from 24 to 34 °C. The results were plotted in Fig. 3. This figure clearly showed that at any fixed D, the ethanol yield increased with an increase of pH up to a certain limit and then it remained almost constant or decreased a little. This result was the same as the 3.2.1 part. In the process of ethanol fermentation with algae as raw material, the initial pH value should not be too low to avoid affecting the activity of enzyme such as alginate lyase.
The Optimal Fermentation Condition
Through the optimization design of the ethanol production process by Design Expert software, the optimal fermentation condition was obtained and the theoretical ethanol yield was calculated. The optimal values of the key parameters in the fermentation process were as follows: KH2PO4 dosage of 4.7 g.L−1, initial pH of 6, fermentation temperature of 28 °C and fermentation time of 99 h. The theoretical value of ethanol yield calculated by the formula was 0.269 g.g−1 (ethanol to algae). Under the optimal experimental conditions obtained by the software, three parallel experiments were carried out to detect the ethanol concentration, and the average ethanol yield was 0.268 g.g−1(ethanol to algae) which was close to the theoretical prediction. The experimental results confirm the reliability of the model. And it was also shown that the Box–Behnken model was suitable for the optimization of the ethanol fermentation process which can also be used with other raw materials. In the previous reports, some researchers carried out ethanol fermentation experiments utilizing brown-algae as raw materials. For example, Pichia stipitis (KCTC7228) and Debaryomyces occidentalis (KCTC7196) were used for fermentation, but the ethanol yield was only 0.029 and 0.1086 g.g−1 algae respectively [30]. By constructing the engineered microbial platform, the enzyme for the transfer and metabolism of alginate was encoded, and the ethanol yield reached 0. 281 g.g−1 algae [15]. And compared with our previous research [27], this ethanol yield of Colpomenia sinuosa was a little higher than that of Laminaria japonica without pretreatment. In addition, the by-product residue after ethanol fermentation can be developed as seaweed fertilizer. On the other hand, the ethanol yield of this microorganism with alginate as substrate was 0.154 g.g−1(ethanol to sodium alginate) [27], which was much lower than the ethanol yield with Colpomenia sinuosa as substrate. Possible two reasons were as follows. Microorganisms can utilize other sugar components in algae, and there might be a cooperative mechanism in the metabolism of alginate and mannitol. Some researchers reported that for some microorganisms, the metabolism of mannitol produced excessive reducing capacity, but compared with mannitol, the production of ethanol by alginate fermentation requires two molecules of reduction capacity per unit molecule [11, 15]. Therefore, the metabolic pathway of alginate can offset the excess reduction capacity produced by mannitol metabolism.
The Alginate Lyase Production During the Fermentation
At present, the ethanol metabolism mechanism of the alginate fermentation strain Meyerozyma guilliermondii is still not clear. Our previous studies showed that this microorganism can produce extracellular alginate lyase to degrade alginate into oligomers and then the oligomers can enter the microbial cells and be metabolized [10]. Therefore, alginate lyase may be the key enzyme in the process of ethanol fermentation. In order to study the ethanol fermentation process of algae, under the optimal fermentation condition (KH2PO4 dosage of 4.7 g.L−1, initial pH of 6 and reaction temperature of 28 °C) the enzyme activity of alginate lyase was measured in the fermentation broth, and the result was compared with the enzyme activity of alginate lyase in the fermentation broth with alginate (without pretreatment) as the substrate instead of algae under the same condition, to analyze the enzyme production of yeast in the fermentation process of algae. The result was shown in Fig. 4. Alginate lyase can be produced with alginate or algae as the substrate, but the enzyme production of algae was slower than that of alginate. The reason may be that other sugar components in algae were first utilized by yeast. And the maximum enzyme activity with algae as substrate was only slightly lower than with alginate as substrate. At the same time, the alginate lyase activity was about 46.42 U.mL−1, compared to 12.79 U.mL−1 in the reference [31], alginate lyase of Meyerozyma guilliermondii was much higher, which indicated that this strain might be a promising strain to convert different kind of algae to bio-ethanol.
Conclusion
This study provides an effective way to realize the production of bioethanol from Colpomenia sinuosa by an alginate fermentation strain Meyerozyma guilliermondii, which can expand the raw materials for bioethanol production. The ethanol production process mainly included two stages: biomass pretreatment and fermentation. The heating pretreatment method provided in this study has the advantages of simple process and easy industrial operation. The soluble sugar produced by the pretreatment can promote the fermentation. In addition, the experimental design of Box-Behnken was used to optimize the parameters of the fermentation process and explore the effect of various factors on the fermentation process. The obtained model was reliable and the best fermentation conditions were obtained, as follows: KH2PO4 dosage of 4.7 g.L−1, initial pH of 6, reaction temperature of 28 °C and fermentation time of 99 h. The ethanol yield reached 0.268 g.g−1, which was much higher than the ethanol yield with alginate as substrate. The alginate lyase production during the algae fermentation was also discussed and compared with the alginate lyase production during the alginate fermentation process. Alginate lyase can be produced with alginate or algae as the substrate, but the enzyme production of algae was slower than that of alginate. This may be related to the utilization of other sugars in the algae. The maximum alginate lyase activity was about 46.42 U.mL−1 which indicated that this yeast might be a promising strain that converts different kind of algae to bio-ethanol. In the follow-up research, the specific mechanism of alginate metabolism to ethanol and the co-utilization mechanism of alginate and mannitol by yeast will be further studied, and various applications of by-products produced in the fermentation process will be further developed.
Data Availability
Please contact author for data requests.
References
Sudhakar K, Mamat R, Samykano M, Azmi WH, Ishak WFW, Yusaf T (2018) An overview of marine macroalgae as bioresource. Renew Sust Energ Rev 91:165–179. https://doi.org/10.1016/j.rser.2018.03.100
Zhang W, Zhang J (2018) The alginate fermentation strain Pantoea sp. F16-PCAi-T3P21 and ethanol production. Energy Source Part A 40:394–399. https://doi.org/10.1080/15567036.2013.844213
Itiki R, Chowdhury PR (2020) Fast deployment of COVID-19 disinfectant from common ethanol of gas stations in Brazil. Health Policy Technol 9:384–390. https://doi.org/10.1016/j.hlpt.2020.07.002
Purohit HJ (2019) Aligning microbial biodiversity for valorization of biowastes: conception to perception. Indian J Microbiol 59:391–400. https://doi.org/10.1007/s12088-019-00826-w
Fernandes AMO, Garcia NFL, Fonseca GG, Leite SR, da Paz MF (2020) Evaluation of the fermentative capacity of Saccharomyces cerevisiae CAT-1 and BB9 strains and Pichia kudriavzevii BB2 at simulated industrial conditions. Indian J Microbiol 60:494–504. https://doi.org/10.1007/s12088-020-00891-6
e Oliveira M, Da CTB, Rosentrater KA (2021) Environmental and economic analysis of low-moisture anhydrous ammonia (LMAA) as a pretreatment for cellulosic ethanol production. J Clean Prod 315:128173. https://doi.org/10.1016/j.jclepro.2021.128173
Patel H, Chapla D, Shah A (2017) Bioconversion of pretreated sugarcane bagasse using enzymatic and acid followed by enzymatic hydrolysis approaches for bioethanol production. Renew Energy 109:323–331. https://doi.org/10.1016/j.renene.2017.03.057
Tinco D, Genier H, Silveira W (2021) Technology valuation of cellulosic ethanol production by Kluyveromyces marxianus CCT 7735 from sweet sorghum bagasse at elevated temperatures. Renew Energy 173:188–196. https://doi.org/10.1016/j.renene.2021.03.132
Alfonsín V, Maceiras R, Gutiérrez C (2019) Bioethanol production from industrial algae waste. Waste Manage 87:791–797. https://doi.org/10.1016/j.wasman.2019.03.019
Zhang W, Bao L (2018) Enzyme detection and metabolic process tracking of ethanol fermentation by a natural alginate fermentation strain. Braz Arch Biol Techn 61:e18160418. https://doi.org/10.1590/1678-4324-2018160418
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: current status and future applications. Renew Sustain Energy Rev 90:316–335. https://doi.org/10.1016/j.rser.2018.03.067
Khoo KS, Chia WY, Chew KW, Show PL (2021) Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J Microbiol 61:262–269. https://doi.org/10.1007/s12088-021-00924-8
Sudhakar MP, Jegatheesan A, Poonam C, Perumal K, Arunkumar K (2017) Biosaccharification and ethanol production from spent seaweed biomass using marine strain and yeast. Renew Energy 105:133–139. https://doi.org/10.1016/j.renene.2016.12.055
Hou X, Hansen JH, Bjerre AB (2015) Integrated bioethanol and protein production from brown seaweed Laminaria digitate. Bioresour Technol 197:310–317. https://doi.org/10.1016/j.biortech.2015.08.091
Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CNS, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A, Kashiyama Y, Baker D, Yoshikuni Y (2012) An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308–313. https://doi.org/10.1126/science.1214547
Chu YJ, Kim HS, Kim MS, Lee EY, Kim HS (2020) Functional characterization of a novel oligoalginate lyase of Stenotrophomonas maltophilia KJ-2 using site-specific mutation reveals bifunctional mode of action, possessing both endolytic and exolytic degradation activity toward alginate in seaweed biomass. Front Mar Sci 7:420. https://doi.org/10.3389/fmars.2020.00420
Takagi T, Yokoi T, Shibata T, Morisaka H, Kuroda K, Ueda M (2016) Engineered yeast whole-cell biocatalyst for direct degradation of alginate from macroalgae and production of non-commercialized useful monosaccharide from alginate. Appl Microbiol Biot 100:1723–1732. https://doi.org/10.1007/s00253-015-7035-x
Ji SQ, Wang B, Lu M, Li FL (2016) Direct bioconversion of brown algae into ethanol by thermophilic bacterium Defluviitalea phaphyphila. Biotechnol Biofuels 9:81. https://doi.org/10.1186/s13068-016-0494-1
Zhang W, Xia X, Zhang Z (2019) Alginate Lyase of a novel algae fermentation strain. Chem Biochem Eng Q 33:125–131. https://doi.org/10.15255/CABEQ.2018.1291
Usov A, Smirnova GP, Kamenarska Z, St D-K, Stefanov KL, Popov SS (2004) Polar constituents of brown seaweed Colpomenia peregrina (Sauv.) hamel from the black Sea. Russ J Bioorg Chem 32:161–167. https://doi.org/10.1023/B:RUBI.0000023102.04368.3d
Manam VK, Subbiah M (2020) Biosynthesis and characterization of silver nanoparticles from marine macroscopic brown seaweed Colpomenia sinuosa (Mertens ex Roth) derbes and solier. J Adv Chem Sci 6:663–666. https://doi.org/10.30799/jacs.219.20060101
Singhvi MS, Deshmukh AR, Kim BS (2021) Cellulase mimicking nanomaterial-assisted cellulose hydrolysis for enhanced bioethanol fermentation: an emerging sustainable approach. Green Chem 23:5064–5081. https://doi.org/10.1039/D1GC01239H
Guo L, Lv M, Wang S, Lu B, Lu X (2010) Determination of total sugar from cherry wine by phenol-sulfuric acid method. Food Res Develop 31:130–131. https://doi.org/10.3969/j.issn.1005-6521.2010.06.038
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:420–428. https://doi.org/10.1021/ac60147a030/
Williams M, Reese D (1950) Colorimetric determination of ethyl ethanol. Anal Chem 22:1556–1561. https://doi.org/10.1021/ac60048a025
Zhang W, Ren X, Zhang Q, Qiu C, Fan B (2019) Application of natural mixed bacteria immobilized carriers to different kinds of organic wastewater treatment and microbial community comparison. J Hazard Mater 377:113–123. https://doi.org/10.1016/j.jhazmat.2019.05.068
Zhang W, Zhang J, Cui H (2014) The isolation and performance studies of an alginate degrading and ethanol producing strain. Chem Biochem Eng Q 28:391–398. https://doi.org/10.15255/CABEQ.2013.1888
Li H, Zhao G, Niu S, Luan Y (2007) Technologic parameter optimization of gas quenching process using response surface method. Comput Mater Sci 38:561–570. https://doi.org/10.1016/j.commatsci.2006.03.014
Zhang L, Zhao B, Liu CJ, Yang E (2020) Optimization of biosynthesis conditions for the production of exopolysaccharides by Lactobacillus plantarum SP8 and the exopolysaccharides antioxidant activity test. Indian J Microbiol 60:334–345. https://doi.org/10.1007/s12088-020-00865-8
Lee SM, Lee JH (2012) Ethanol fermentation for main sugar components of brown-algae using various yeasts. J Ind Eng Chem 18:16–18. https://doi.org/10.1016/j.jiec.2011.11.097
Chu H, Tang J (2008) Study on culture conditions for alginase production by alginate degrading bacterium Gracillibacillus sp. A7. Mar Sci 32:93–96. https://doi.org/10.1007/s11676-008-0012-9
Acknowledgements
The authors gratefully acknowledge the financial supports from Natural Science Foundation of Zhejiang Province (LQ20B060004), and Zhejiang Shuren University Basic Scientific Research Special Funds (2021XZ016).
Funding
This research was supported by Natural Science Foundation of Zhejiang Province under Grant No. LQ20B060004 and Zhejiang Shuren University Basic Scientific Research Special Funds (2021XZ016).
Author information
Authors and Affiliations
Contributions
Conceptualization: WZ; Data curation: WZ and Y M; Writing-original draft: WZ; Writing-review & editing: WZ and MW; Methodology: ZL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Mao, Y., Liu, Z. et al. Ethanol Production from Colpomenia sinuosa by an Alginate Fermentation Strain Meyerozyma guilliermondii. Indian J Microbiol 62, 112–122 (2022). https://doi.org/10.1007/s12088-021-00985-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-021-00985-9