Abstract
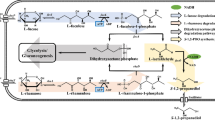
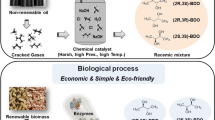
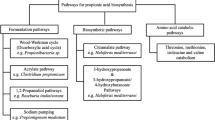
1,2-Propanediol (propylene glycol) is an existing commodity chemical and can be produced from renewable resources using microbes. By virtue of being a natural product, relevant biochemical pathways can be harnessed into fermentation processes to produce 1,2-propanediol. In the present review, the chemical process and different biological strategies for the production of 1,2-propanediol are reviewed and compared with the potentials and limitations of all processes. For the successful commercial production of this diol, it is necessary to establish the metabolic pathways and production hosts (microorganisms), which are capable of delivering final product with high yields and volumetric productivity. Three pathways which have been recognized for 1,2-propanediol production are discussed here. In the first, de-oxy sugars like fucose and rhamnose are used as the carbon sources, while in the other route, the glycolytic intermediate-dihydroxyacetonephosphate (DHAP) is used to produce 1,2-propanediol via the formation of methylglyoxal. A new pathway of 1,2-propanediol production by lactic acid degradation under anoxic conditions and the enzymes involved is also discussed. The production of this diol has gained attention because of their newer applications in industries such as polymers, food, pharmaceuticals, textiles, etc. Furthermore, improvement in fermentation technology will permit its uses in other applications. Future prospect in the light of the current research and its potential as a major bulk chemical are discussed.
Similar content being viewed by others
References
Behr A, Eilting J, Irawadi K, Leschinski J and Lindner F (2008) Improved utilization of renewable resources: New important derivatives of glycerol. Green Chemistry 10:13–30
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R and Tschaplinski T (2006) The path forward for biofuels and biomaterial. Science 311:484–489
Hartlep M, Hussman W, Prayitno N, Meynial-Salles I and Zeng AP (2002) Study of two-stage processes for the microbial production of 1,3-propanediol from glycerol. Appl Microbiol Biotechnol 60:60–66
Chotani G, Dogde T, Hsu A, Kumar M, La Duca R, Trimbur D, Weler W and Sanford K (2000) The commercial production of chemicals using pathway engineering. Biochim Biophys Acta 1543:434–455
Cameron DC, Altaras NE, Hoffman ML and Shaw AJ (1998) Metabolic engineering of propanediol pathway. Biotechnol Prog 14:116–125
Bennett GN and San KY (2001) Microbial formation, biotechnological production and applications of 1,2-propanediol. Appl Microbiol Biotechnol 55:1–9
Altaras NE and Cameron DC (1999) Metabolic engineering of a 1,2-propanediol pathway in E. coli. Appl Environ Microbiol 65:1180–1185
Lenth CW and Puis RNDu (1945) Polyhydric alcohol production by hydrogenolysis of sugars in the presence of copper-aluminium oxide. Ind Eng Chem Res 37:152–157
Martin AE and Murphy FH (1994) Propylene glycols. In: Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 17, 4th edition, Kroschwitz JI (Ed.), Wiley, New York, pp 715–726
Fryzuk MD and Bosnich B (1978) Asymmetrical synthesis: An asymmetric homogenous hydrogenation catalyst which breeds its own chirality. J Am Chem Soc 100:5491–5494
Kometani T, Yoshii G, Takeuchi Y and Matsuno T (1993) Preparation of chiral 1,2-alkanediols with baker’s yeast-mediated oxidation. Chem Lett 12:2123–2124
Dasari MA, Kiatsimkul PP, Sutterlin WR and Suppes GJ (2005) Low pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A-Gen 281:225–231
Braca G, Raspolli Galletti AM and Sbrana G (1991) Anionic ruthenium iodocarbonyl complexes as selective dehydroxylation catalysts in aqueous solution. J Organomet Chem 417:41–49
Herrera S (2004) Industrial biotechnology - a chance at redemption. Nat Biotechnol 22(6):671–678
Suzuki T and Onishi H (1968) Aerobic dissimilation of α-Rhamnose and the production of α-Rhamnoic acid and 1,2-propanediol by yeast. Agr Biol Chem 32:888–893
Badia J, Ros J and Aguilar J (1985) Fermentation mechanism of fucose and rhamnose in Salmonella typhium and Klebsiella pneumoniae. J Bacteriol 161:435–437
Enebo L (1954) Studies in cellulose decomposition by an anaerobic thermophilic bacterium and two associated non-cellulolytic species. Viktor Pettersons Bokindustrie Akuebolag. Stockholm
Turner KW and Roberton AM (1979) Xylose, arabinose, and rhamnose fermentation by Bacteriodes ruminicola. Appl Environ Microbiol 38(1):7–12
Boronat A and Aguilar J (1981) Metabolism of L-fucose and L-rhamnose in Escherichia coli: differences in induction of propanediol oxidoreductase. J Bacteriol 147:181–185
Tran-Din K and Gottaschalk G (1985) Formation of D(-)-1,2- propanediol and D(-)-lactate from glucose by Clostridium sphenoides under phosphate limitation. Arch Microbiol 142:87–92
Cameron DC and Cooney CL (1986) A novel fermentation: The production of (R)-1,2-propanediol and acetol by Clostridium thermosaccharolyticum. Biores Technol 4:651–654
Sanchez Rivera F, Cameron DC and Cooney CL (1987) Influence of environmental factors in the production of 1,2-propanediol by Clostridium thermosaccharolyticum. Biotechnol Lett 9:449–454
Altaras NE, Etzel MR and Cameron DC (2001) Conversion of sugar to 1,2-propanediol by Thermoanaerobacterium thermosaccharolyticum HG-8. Biotechnol Prog 17:52–56
Dowd MK, Johnsen SL, Cantarella L and Reilly PJ (1994) Low molecular weight organic composition of ethanol silages from sugarcane molasses, citrus waste and sweet whey. J Agric Food Chem 42:283–288
Hoffman ML (1999) Metabolic engineering of 1,2-propanediol product in Saccharomyces cerevisiae, Ph.D thesis, University of Wisconsin - Madison
Obradors N, Badia J, Baldoma L and Aguilar J (1988) Anaerobic metabolism of the L-Rhamnose fermentation product 1,2-propanediol in Salmonella typhimurium. J Bacteriol 170(5):2159–2162
Veiga da Cunha M and Foster MA (1992) Sugar-glycerol co fermentations in Lactobacilli: The fate of lactate. J Bacteriol 174:1013–1019
Elferink SJWHO, Krooneman J, Gottschal JC, Spoelstra SF, Faber F and Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Appl Environ Microbiol 67(1):125–132
Boronat A and Aguilar J (1979) Rhamnose-induced propanediol oxidoreductase in Escherichia coli: purification, properties and comparison with the fucose-induced enzyme. J Bacteriol 140:320–326
Lin ECC (1980) Conversion of reductases to dehydrogenase by regulatory mutations. In: Trends in the Biology of Fermentation for Fuels and Chemicals. Hollaender A (Ed.), Plenum Press, New York, London, pp 305–313
Weimer PJ (1983) Fermentation of 6-deoxyhexoses by Bacillus maceran. Ann Meet Am Soc Microbiol 10:241
Cooper RA (1975) The separation of 2,4-dinitrophenylhydrazones by thin layer chromatography. J Chromatogr 20:528–540
DeLey J and Kersters K (1964) Oxidation of aliphatic glycols by acetic acid bacteria. Bacteriol Rev 28:164–180
Tanaka Y, Fujii K, Tanaka A and Fukui S (1975) Metabolism of 1,2-propanediol in a soil bacterium. J Ferment Technol 53:354–362
Murata K, Fukuda Y, Watanabe K, Saikusa T, Shimosaka M and Kimura A (1985) Charaterization of methyglyoxal synthase in Sacchromyces cerevisiae. Biochem Biophys Res Commun 131(1):190–198
Nakamura K, Kondo S, Kawai Y, Nakajima N and Ohno A (1997) Amino acid sequence and characterization of aido-keto reductase from bakers’ yeast. Biosci Biotechnol Biochem 61:375–377
Simon ES, Whitesides M, Cameron DC, Weitz DJ and Cooney, CL (1987) A combined microbial/chemical synthesis of (+)-(R)-methyloxirane having high enantiomeric excess. J Org Chem 52:4042–4044
Forage R and Lin ECC (1982) dha system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB418. J Bacteriol 151:591–599
Hacking AJ and Lin EC (1976) Disruption of the fucose pathway as a consequence of genetic adaptation of propanediol as a carbon source in E. coli. J Bacteriol 126:1166–1172
Takagi Y and Sawada H (1964) The metabolism of L-rhamnose in E. coli L-rhamnose isomerase. Biochim Biophys Acta 92:10–17
Heath EC and Ghalambor MA (1962) The metabolism of fucose II: the purification and properties of L-fucolose kinase. J Biol Chem 237:2423–2426
Ghalambor MA and Heath EC (1962) The metabolism of fucose II: The enzymatic cleavage of L-fucolose-1-phosphate. J Biol Chem 237:2427–2433
Hacking AJ and Lin EC (1977) Regulatory changes in the fucose system associated with the evolution of a catabolic pathway for propanediol in Escherichia coli. J Bacteriol 130:832–838
Beckmann BJ and Low KB (1980) Linkage of E. coli K12. Microbiol Rev 44:1–36
Huang KX, Rudolph FB and Bennet GN (1999) Characterization of methylglyoxal synthase from Clostridium acetobutylicum ATCC 824 and its use in the formation of 1,2-propanediol. Appl Environ Microbiol 65(7):3244–3247
Hopper DJ and Cooper RA (1972) The purification and properties of Escherichia coli methyglyoxal synthase. Biochem J 128:321–329
Tsai PK and Gracy RW (1976) Isolation and characterization of crystalline methylglyoxal synthase from Proteus vulgaris. J Biol Inorg Chem 251:364–367
Wong CH (1994) Enzymes in Synthetic Organic Chemistry. Elsevier Science Ltd., London, United Kingdom
Yoo DS, Lee JW and Yun HS (2008) Optimization of production of 1,2-propanediol in Saccharomyces cerevisiae by using dual vector system. J Biotechnol 136S:S345–S355; Abstracts V1-P-019
Noh YH, Kim SK, Park BJ, Lee JW and Yun HS (2008) Optimization of production of 1,2-propanediol from Saccharomyces cerevisiae by using dual promoter vector system. J Biotechnol 136S:S290–S344; Abstracts V1-P-106
Su W, Chang Z, Gao K and Wei D (2004) Enantioselective oxidation of racemic 1,2-propanediol to D-(-)-lactic acid by Gluconobacter oxydans. Tetrahedran Asymmetry 15:1275–1277
Bauer MC, Weiss DJ and Perman V (1992) Hematological alterations in kitten induced by 6 and 12% dietry propylene glycol. Vet Hum Toxicol 34:127–131
Mailhes JB, Young D and London SN (1997) 1,2-Propanediol-induced premature centromere separation in oocytes and aneuploidy in one-cell zygote. Biol Reprod 57(1):92–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxena, R.K., Anand, P., Saran, S. et al. Microbial production and applications of 1,2-propanediol. Indian J Microbiol 50, 2–11 (2010). https://doi.org/10.1007/s12088-010-0017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-010-0017-x