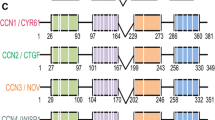
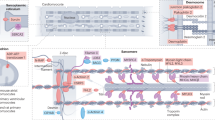
Abstract
The Fifth International Workshop on the CCN Family of Genes was held in Toronto October 18–22, 2008. This bi-annual workshop provides a unique opportunity for the presentation and discussion of cutting edge research in the CCN field. The CCN family members have emerged as extracellular matrix associated proteins which play a crucial role in cardiovascular and skeletal development, fibrosis and cancer. Significant progress has been made in the development of model systems to tease apart the CCN signalling pathways in these systems. Results presented at the conference suggest that targeting these pathways now shows real promise as a therapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Fifth International Workshop on the CCN Family of Genes was held in Toronto October 18–22, 2008. The meeting was organised by Herman Yeger, Bernard and Annick Perbal and provided an excellent environment for discussion and interaction amongst a diverse and enthusiastic group of researchers.
The Journal of Communication and Cell Signalling has now been launched as the official journal of the International CCN Society. The publishers, Springer Science & Business Media, sponsored the opening plenary session of the conference and also three scholarships for young investigators to attend the meeting. The first Springer Award for outstanding research in this field was presented by Peter Butler (Springer) to Professor Paul Bornstein for his work on matricellular proteins. Professor Bornstein gave a stimulating talk focussed on the concept of ‘dynamic reciprocity’ and presented his studies using thrombospondin (TSP-1 and TSP-2) null mice. Opening remarks by Professor Perbal coupled with Professor Bornstein’s lecture set the stage nicely for the meeting to follow.
The first session of the meeting on CCN Structure/ Function and Expression opened with a presentation by Dr Ravi Acharya (Bath, England). Dr Acharya examined the protein structure of the CCN family and discussed how this might contribute to the functional differences between family members. By modelling the three dimensional structure of the domains he provided insight as to how this might influence interaction with other key molecules. This was followed by a series of presentations on CCN2 gene regulation lead by Dr Satoshi Kubota (Okayama, Japan) who described the characterisation of Nucleophosmin/B23 as a regulator of CCN2 in chicken chondrocytes. Nucleophosmin has the ability to shuttle between the nucleus and the cytoplasm and Satoshi presented extensive experiments demonstrating both transcriptional (nuclear) and post-transcriptional (cytoplasmic) regulation of CCN2. Further work from this group, presented by Dr Ogawara, demonstrated that mi-RNA 18a acts on CCN2 via the 3’-UTR and regulates human chondrocytic differentiation. This work now introduces a new level of CCN regulation that should get increasing attention. In a change of cellular context, Dr Cabello-Verrugio (Santiago, Chile) presented work on the regulation of CCN2 by TGF-b and LPA in skeletal muscle cells in parallel with other studies from his lab suggesting dependency on decorin and involvement of the endocytic receptor LRP-1. He used the C2C12 myoblast cell line to show a dose-dependent induction of CCN2 where the induction is mediated classically through TGFbR1 and SMAD2/3.
The final paper in this session was given by Dr Yasuda (Boston, USA) and described the use of ChIP on CHIP analysis to identify a SOX9 binding site in the promoter region of CCN2.
Brahim Chaqour (New York, USA) described elegant studies to examine the mechanical regulation of CCN1 in smooth muscle cells. Myocardin related transcription factor (MRTF-A) was shown to shuttle between the nucleus and the cytoplasm being localised in the cytoplasm in unstimulated cells and accumulating in the nucleus of mechanically stimulated cells. He showed that nuclear MRTF-A acts in concert with P300/CBP to regulate CCN1. Periostin is not a member of the CCN family but is a novel secreted matricellular protein with a similar expression pattern to CCN2. Douglas Hamilton (Ontario, Canada) described experiments showing that periostin expression was increased in mechanically stressed skin fibroblasts and was also increased in cutaneous wound repair. Periostin seems to become extracellular in various pathologies and its mode of action and expression suggest that it may have similarities to the CCN family. Interestingly, periostin binds to CCN3. Lan Wei (Boston, USA) constructed a series of plasmids expressing each domain of CCN5 alone and in combinations both with and without the secretory peptide. By using fluorescently tagged constructs they were able to show that non-secreted forms of the Von Willebrand type C (V) domain alone, Thrombospondin type-1 domain (T) alone and IV, VT and IVT domains were imported into the nucleus; the same constructs containing an N-terminal signal peptide were not detected. They are currently applying monoclonal antibodies against specific domains of CCN5 to further elucidate its mode of action. This session finished with a return to the topic of CCN2 regulation. Hirokazu Okada (Saitama, Japan) identified a 20bp region in the mouse CCN2 promoter which is bound by PolyADP-Ribose Polymerase-1 (PARP-1) and named it PARP Binding Element (PBE). Since PARP-1 is a higher order modifier of histones and CCN2 is the main mediator of the pro-fibrotic effects of TGF-β in fibrotic kidneys, this may open up new anti-fibrotic therapeutic targets for this disorder.
The session on Osteogenesis and Chondrogenesis was opened by Professor Masaharu Takigawa (Okayama, Japan) who described a transgenic mouse model in which the CCN2 gene was overexpressed in cartilage under the control of the type II collagen promoter. He showed that this lead to accelerated endochondral ossification by promoting proliferation and differentiation of chondrocytes. He also compared wild-type and CCN2 null mice and showed that CCN2 is also required for normal intramembranous bone development. This theme was carried on by a presentation from Fayez Safadi (Philadelphia, USA) who has developed a transgenic mouse model in which CCN2 is overexpressed in cells of the osteoblast lineage. By comparing moderate to high level overexpressing mice he was able to show that moderate levels of CCN2 promote bone formation whilst high levels have an opposite effect and promote osteoclast formation. Faith Hall-Glenn (Los Angeles, USA) described a further mouse model in which CCN1 and CCN2 were specifically knocked out in cartilage. Cartilage specific loss of CCN1 is perinatal lethal although the skeletons of the mice have only minor alterations whilst the double knockout animals have a much more severe phenotype. The phenotypes of the animals developed by Takigawa and Hall-Glenn were quite different reflecting the different strategies used to control gene expression. These animal models provide exciting new tools to investigate the functional roles of the CCN proteins. Ken-ichi Katsube (Tokyo, Japan) constructed CCN3 deletion mutants lacking the CT domain (del CT) and used these to study NOTCH and BMP signalling in the mouse osteogenic cell line, Kusa-A1. The CT domain is responsible for binding NOTCH yet the delCT mutant was still able to activate downstream NOTCH signalling pathways and was able to bind BMP. These results suggest that the inhibitory effect of CCN3 on osteogenesis is mediated by both NOTCH and BMP signalling pathways. Andrew Leask (Ontario, Canada) grew mesenchymal cells in a micromass model culture system for his studies on chondrogenesis. FAK/src signalling mediates cell adhesive properties and inhibition of this pathway lead to increased expression of CCN2 and chondrogenic matrix associated genes in wild type cells; CCN2 null cells did not respond. These results suggest that CCN2 operates downstream of FAK/src and that loss of FAK/src is critical for chondrocytic differentiation.
Kruppel-like factor 15 (KLF-15) is expressed in both cardiomyocytes and fibroblasts and is reduced by pro-hypertrophic stimuli both in vitro and in vivo. Mukesh Jain (Cleveland, USA) used a rat ventricular fibroblast cell model to show that KLF-15 inhibits CCN2 by preventing recruitment of P/CAF to the promoter region. He also generated KLF-15 null mice and found increased levels of CCN2 and collagen in the heart as a result of mechanical stress. These results suggest that KLF-15 plays a key role in the regulation of cardiac stress. Mark Erwin (Toronto, Canada) presented some fascinating studies using canine models to explore biological therapies for degenerative disc disease. Mongrel dogs which do not get disc problems retain a notochordal cell rich region, and these cells secrete CCN2 which can then upregulate aggrecan expression in chondrocytes. In contrast, Beagles are prone to disc degeneration similar to ageing humans. This is likely to be a promising non-rodent animal model for the CCN field. Karen Lyons (Los Angeles, USA) used a CCN2-/- mouse model and mice with CCN2 specifically knocked out in endothelial cells to look for effects on angiogenesis. She showed that the vascular system develops normally in these mice initially but that vascular remodelling is defective, probably due to defective basement membrane assembly. Of further relevance here is the disruption in pericyte/endothelial cell interactions with broader implications for stem cell biology. Ursula Kees (Perth, Australia) has found markedly elevated levels of CCN2 in paediatric acute lymphoblastic leukaemia (ALL). She presented data showing that CCN2 may be involved in the interaction of the ALL cells with the bone marrow microenvironment and is currently investigating the underlying mechanisms involved. Gene enrichment analysis suggested multiple genes upregulated in ALL as in other cancers.
The first of the pathobiology sessions started with a series of presentations examining the role of CCN proteins in normal and wounded skin. Laure Rittie (Michigan, USA) used laser capture micro-dissection coupled with real time PCR and immunocytochemistry to study CCN expression in normal skin and following wounding by thermal ablation. She showed very clearly that the CCN proteins are expressed in a cell type and stage specific manner during wound healing. She also observed CCN expression in nuclei during injury and a marked decrease in CCN3 after wounding and return to normal levels in the healing phase thus supporting the notion of CCN proteins operating alternately and in balance. Following on from this Andrew Leask specifically examined CCN2 using a novel transgenic knock-in mouse model with a GFP gene inserted between the endogenous CCN2 promoter and gene. Using this strategy Andrew was able to show that, on wounding, CCN2 induction parallels the appearance of myofibroblasts and that pericytes also express CCN2 and contribute to myofibroblast activity. This data further begs the question of whether reactive mesenchymal phenotypes all exploit CCN2 for repair processes. Taihao Quan (Michigan, USA) presented studies on UV and chronologically aged skin showing that CCN1 plays a role in aberrant dermal collagen homeostasis. The thinking is that changes in CCN1 modify the TGFb mediated effects on collagen synthesis and thereby structurally change skin. Interestingly, he also found that retinol had the potential to reverse many of these effects consistent with its application in anti-ageing skin care products. Kirsten Bielefeld (Toronto, Canada) gave an excellent presentation on her doctoral studies of β-catenin during wound healing. She used primary dermal fibroblasts to show that the extracellular matrix acts as a feedback loop to regulate β-catenin during wound repair. DEL1, a further matricellular protein with an expression pattern similar to CCN1 and CCN2, was shown to enhance bone fracture healing (Yang, Stanford, USA). In contrast to the CCN1 and CCN2 mutants, DEL1 knockout mice have normal skeletons but heal fractures with less bone. This may reflect a role for DEL1 in preventing premature apoptosis of the hypertrophic cartilage during endochondral ossication. Cutler (Maryland, USA) used a mouse mammary epithelial cell line (HC-11) to examine the role of CCN2 during lactogenic differentiation. She showed that CCN2 levels increased more than ten fold during differentiation, CCN2 enhances HC-11 differentiation, blocked by CCN2 siRNA, and that effects are mediated via activation of the β-1 integrin mediated adhesion complexes and integrin dependent signalling pathways. Shakil Ahmed (Oslo) used CCN2 transgenic mice to investigate the role of CCN2 in heart disease. CCN2 was shown to act as a survival factor in these mice resulting in reduced infarct size and improved recovery. Apparently the cardioprotection exhibited by CCN2 is mediated by a complex regulatory network. Stephen Twigg (Sidney, Australia) followed on from this with studies of CCN2 in diabetic cardiomyopathy. In contrast to the previous study, CCN2 was found to mediate the adverse effects of high glucose and free fatty acids in the H9C2 cardiomyocyte cell line. Treatment with a specific trkA inhibitor, K252a, blocked the effects of CCN2 on hypertrophy and apoptosis, an observation supporting the previous report by Wahab et al. (2005), suggesting that CCN2 is acting via the trkA pathway. The search for new biomarkers in systemic sclerosis was explored by Robert Lafyatis (Boston, USA) who found that TGF-β regulated genes, including CCN2, are increased in the skin of patients with scleroderma and that some of these genes (COMP and TSP1) correlated with a modified Rodnan skin score. Using the tight skin mouse (Tsk) model he also found that CCN3 was highly expressed and postulated that it counter-regulates fibrillin matrix fibre assembly and deposition.
Takako Hattori (Okayama, Japan) used a combination of in vitro and in vivo studies to show that SOX9 binds to the enhancer region of the CCN2 gene. She then used mouse models in which CCN2 was specifically overexpressed in skin or cartilage to demonstrate a stimulatory feedback loop involving CCN2, SOX9 and aggrecan. CCN2 colocalized with aggrecan on the cell surface. Joshua Russo (Boston, USA) described a new model system developed to study leiomyoma in vivo. Fresh human fibroid tissue is broken up and resuspended in a matrigel/ collagen I mixture before subcutaneous injection into mice. Following hormone supplementation, human smooth muscle cells grow out of the injection site where they acquire a blood supply through angiogenesis. These mice provide an excellent system in which to study the effects of CCN5 in fibroids. In addition, in tumor xenograft models he showed that CCN5 slowed down tumor growth with reduction in the tumor vascular architecture. Shiwen (London, England) investigated the mechanisms linking microvascular damage to the fibrogenic system in patients with scleroderma (SSc). He found that endothelin-1 stimulated pericytes (a multipotent phenotype) and fibroblasts to produce CCN2 and collagen, via ERK1/2 mediated signalling, and that pericytes acquire fibroblast markers on long term culture. This suggests that pericytes may contribute to the fibrosis observed in SSc and must be considered when developing new treatment strategies. Enrique Brandan (Chile) used a mouse model for Duchenne muscular dystrophy in which exercise protocols induce fibrosis and also lead to an increase in CCN2. He used this system to show that the proteoglycan, decorin, can interact with CCN2 inhibiting the fibrotic action. Gingival fibrosis is a clinical problem which often occurs as a side effect of medication such as cyclosporin. In the first of two presentations from Boston, Alpdogan Kantarci showed that CCN2 is increased at both the mRNA and protein levels in drug induced gingival fibrosis and examined the role of CCN2 in promoting fibrotic lesions. Philip Trackman then described some beautiful studies in which he delineated the mechanisms underlying the problem and designed a dual pronged treatment strategy using lovastatin and forskolin to reduce TGF-β stimulated CCN2 levels in gingival cells. Bruce Riser (Chicago, USA) used an in vitro model of renal fibrosis to look for endogenous inhibitors of CCN2 and explore the possibility of interaction with other CCN family members. He found that CCN3 (either provided exogenously or overexpressed) downregulates CCN2 activity in mesangial cells and blocks ECM overaccumulation stimulated by TGFβ thus providing an opportunity for therapeutic intervention. This inverse relationship between CCN3 and CCN2 is in agreement with the recently published results by Kawaki et al. (2008). David Brigstock (Columbus, Ohio) has developed an exciting therapeutic strategy to target fibrosis in a mouse model of hepatic fibrosis and evaluated anti-CCN2 therapy in both a preventative and curative setting (before or after onset of collagen deposition). Liposomes containing CCN2 siRNA, when coated with a synthetic peptide to ensure they homed to activated hepatic stellar cells, proved to be effective as an anti-fibrotic agent. The ready delivery of CCN2 siRNA across multiple tissue barriers opens up possibilities for translational studies in the CCN field. The final presentation of this session was from Margarete Gopelt-Struebe (Erlangen, Germany) who investigated the hypoxia-induced regulation of CCN2. Hypoxia was induced by the DMOG inhibitor of PHD2 and thereby activation of HIF1a. She demonstrated that regulation of CCN2 by hypoxia is cell type dependent and involves the FoxO family of transcription factors. Interestingly, she showed an additive effect of DMOG and TGFb a more complex regulatory situation during injury.
In the spirit of promoting the bridging of two fields, CCN and Matrix, a new feature of the workshop was a special session entitled ‘From Matricellular to Extracellular’ (see http://ccnsociety.com/award.html). Three eminent clinician-scientists from Toronto put the entirety of the conference in to context by presenting the human consequences of dysregulation in the extracellular matrix system. Aleksander Hinek described how defective production of components involved in elastin microfibril assembly contributes to numerous skeletal and vascular disorders. William Cole focused on how studies of rare genetic disorders have led to greater understanding of the genes required for normal development of bone and cartilage. Ren-Ke Li gave an inspirational talk on the potential of myocardial cell therapy. Through detailed studies with animal models he is teasing apart the underlying mechanisms for cell based therapy as an approach to matrix remodelling. Katherine Sodek has just completed her PhD and presented the work she carried out on ovarian cancer. She used a novel 3-D culture system and showed that MT1-MMP and MMP2 contribute to cell motility and matrix degradation whilst treatment with TGFβ stimulated spheroid formation and was associated with increased invasive capacity. This was an excellent session and set the clinical framework of matricellular disorders.
CCN3 came to the fore in the second pathobiology session of the meeting. Vivianna Vallachi (Milan, Italy) found that increased CCN3 expression was associated with poor prognosis in metastatic melanoma. Analysis of CCN3 in cultures of cells from melanoma lesions showed heterogeneous expression of the 46 kDa (mostly cytoplasmic) and 32 kDa (nuclear) proteins but this was not associated with specific CCN3 gene mutations; however, CCN3 polymorphisms were noted. Xenotransplanatation studies in immunodeficient mice showed a higher metastatic potential in CCN3 overexpressing cells and a greater resistance to induction of apoptosis by cancer chemotherapeutic drugs. In contrast, CCN3 expression is downregulated as a result of BCR-ABL kinase activity in Chronic Myeloid Leukaemia (CML; Mc Callum, Belfast, Ireland). Increased CCN3 expression levels resulted in decreasing levels of phosphorylated ERK reducing cell proliferation whilst also increasing levels of cleaved caspase 3 and restoring induction of apoptosis. Primary human CML cells demonstrated growth inhibition in response to recombinant CCN3 which may be important for developing additional therapeutic strategies. Perbal et al. (Paris, France and Bologna, Italy) examined CCN1-3 to evaluate their prognostic value in osteosarcoma and Ewings sarcoma. They found that CCN3 expression was associated with increased attachment, migration and an aggressive phenotype and with an increased risk of recurrence and metastases. A high number of cases expressed a CCN3 variant, lacking the NH3 domain which conferred worse prognosis for patients receiving chemotherapy and radiotherapy. Studies of CCN1 in osteosarcoma (A. Sabile, Zurich, Switzerland) showed it was upregulated in metastatic osteosarcoma cell lines and also in primary tissues from patients. CCN1 induced phosphorylation of Akt and GSK-3b and coincided with localization of p21 in the cytoplasm. This mechanism effectively activates both pro-survival and pro-proliferative pathways. CCN3 co-localises with a core component of gap junction complexes, connexin43 (Cx43). These observations are in agreement with previously published work reporting the co-localisation of CCN3 and Cx43 ( Fu et al. 2004; Gellhaus et al. 2004).Wun-Chey Sin (Vancouver, Canada) found Cx43 is down-regulated in aggressive breast tumours and Cx43 levels positively regulate expression of CCN3. Overexpresson of CCN3 in breast cancer cells inhibited cell growth and was involved in reorganization of the actin cytoskeleton and re-distribution of focal adhesions. Further work on breast cancer was discussed by Ruth Lupu (Rochester, USA). She found that CCN1 expression is correlated with advanced disease in breast cancer and induces a taxol resistant phenotype. CCN1 upregulates expression of the αvβ3 integrin; functional blockade of αvβ3 with a synthetic chemical peptidomimetic of the RGD motif is cytotoxic for CCN1 expressing breast cancer cells. Disrupting the interaction of CCN1 and αvβ3 regained sensitivity to taxol. Zoledronic acid, an aminobisphosphonate, inhibits CCN1 expression and reduces anchorage independent cell growth as well as disrupting vimentin distribution. These findings present a novel therapeutic strategy for targeting metastatic breast cancer. Kallikreins (KLKs) are a multi-gene family of secreted serine proteases. KLKs and kallikrein related peptidases have important implications in regulating cancer cell growth, angiogenesis, invasion and metastasis. Yves Courty (Tours, France) found that KLK12 could cleave CCN1 from the surface of a tumour cell line. He went on to show that all members of the CCN family can be digested with KLKs, notably CCN1 and CCN5 by KLK12 and KLK14, CCN3 by KLK5, 12 and 14. Further work will ascertain the functional CCN properties before and after degradation. The fact that KLK activity released smaller CCN fragments opens up new possible roles for CCN proteins in the multiple disease states where KLKs figure prominently. Finally Sushanta Banerjee (Kansas, USA) presented his investigations of the role of mi-RNA-10b in metastatic breast cancer. CCN5 is expressed in non-invasive breast cancer cell lines whilst it is not detected in invasive breast cancer. Silencing CCN5 expression in MCF7 cells, increased miR-10b expression and increased cell migration and invasiveness. The mechanism driving invasive capacity is thought to involve down-regulation of CCN5 expression causing increased levels of miR-10b and the transcription factor, twist, leading to increased levels of HIF1α and enhanced motility.
The final session of the meeting featured presentations by the Springer Scholarship awardees. Wei Huang (Michigan, USA) constructed a CCN6 deficient human mammary epithelial cell line model and used this to look at the effects on E-cadherin. CCN6 inhibition was associated with decreased E-cadherin expression which was shown to be mediated by upregulation of SNAIL and ZEB1. These observations are important since loss of E-cadherin is associated with de-differentiation, invasion and metastasis. Since CCN3 is associated with Cx43 perhaps CCN proteins play distinct and important roles in organization of cell junction complexes not yet fully appreciated. Further studies in breast cancer were presented by Ingrid Espinoza (Chicago, USA) who examined the role of CCN1 in the hormonal response. She used a mutant CCN1 construct with substitutions in the α6β1 binding domain to support a possible role for CCN1 as a co-activator of ER, involved in the transcriptional activation of proliferative and survival ERE-genes in breast cancer cells. Of relevance here was the data supporting the posited nuclear role for CCN proteins in transcriptional events. Takanori Eguchi (Okayama, Japan) described a novel role for matrix metalloproteinase (MMP)-3 as a promoter of extra-cellular matrix production through CCN2 trans-activation. MMPs are conventionally regarded as extracellular acting proteases but Takanori showed that MMP-3 can be translocated into the nucleus and bind the Transcription Enhancer Dominant In Chondrocytes (TRENDIC) to regulate CCN2. All three presentations were of an extremely high standard and the recipients were to be congratulated.
Lester Lau concluded the meeting with a masterful exposition on CCNs and inflammation, pulling together many strands of interest that permeated the meeting, particularly the exciting new model systems which have been developed and progress towards translational applications. With much to challenge and stimulate us, and after closing remarks by Dr. Yeger and Prof Perbal, Dr. S. Irvine (Belfast, Ireland) extended an enthusiastic invitation to the 6th ICCNS workshop to be held in Belfast, 2010.
References
Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC (2004) CCN3 (NOV) interacts with connexin43 in glioma cells: a possible mechanism of connexion-mediated growth suppression. J Biol Chem 279:36943–36950. doi:10.1074/jbc.M403952200
Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, Winterhager E (2004) Connexin interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem 279:36931–36942. doi:10.1074/jbc.M404073200
Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M (2008) Co-operative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res 23:1751–1764. doi:10.1359/jbmr.080615
Wahab NA, Weston BS, Mason RM (2005) Connective tissue growth factoro CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol 16:340–351. doi:10.1681/ASN.2003100905
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Irvine, A.E., Perbal, B. & Yeger, H. Report on the fifth international workshop on the CCN family of genes. J. Cell Commun. Signal. 2, 95–100 (2008). https://doi.org/10.1007/s12079-009-0036-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-009-0036-8