Abstract
Background
Vessels that encapsulate tumor clusters (VETC) is a novel described vascular pattern different from microvascular invasion (MVI) for patients with hepatocellular carcinoma (HCC). The prognostic value of integrating VETC and MVI (VETC-MVI model) in HCC patients after resection remains unclear.
Methods
From January 2013 to December 2016, 498 HCC patients who underwent curative resection were enrolled from five academic centers and stratified into different groups according to their VETC and MVI statuses. Overall survival (OS), disease-free survival (DFS), and early and late recurrence rates were evaluated.
Results
The patients were divided into four subgroups: VETC−/MVI− (n = 277, 55.6%), VETC−/MVI+ (n = 110, 22.1%), VETC+/MVI− (n = 53, 10.6%), and VETC+/MVI+ (n = 58, 11.6%). The patients in the VETC+/MVI− and VETC−/MVI+ groups had similar long-term outcomes (OS: p = 0.402; DFS: p = 0.990), VETC−/MVI− patients showed the best prognosis, and VETC+/MVI+ patients had the worst prognosis. Further analysis revealed that the VETC-MVI model showed a similar stratification ability for early recurrence but not for late recurrence. The area under the curve values for early recurrence was 0.70, 0.63 and 0.64 for the VETC-MVI model, VETC, and MVI, respectively (VETC-MVI model vs VETC: p < 0.001; VETC-MVI model vs MVI: p = 0.004; VETC vs MVI: p = 0.539). Multivariate Cox regression analysis showed that the VETC-MVI model successfully predicted OS, DFS and early recurrence.
Conclusions
VETC status provides additional discriminative information for patients with either MVI− or MVI+. A combination of VETC and MVI may help classify subtypes and predict the prognosis of HCC patients.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the Cancer Center Institutional Data Access/Ethics Committees of the five academic hospitals involved in this study, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301.
Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–20.
Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65(4):798–808.
Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. J Clin Investig. 2010;120(4):1031–4.
Ding ZB, Shi YH, Zhou J, Shi GM, Ke AW, Qiu SJ, et al. Liver-intestine cadherin predicts microvascular invasion and poor prognosis of hepatitis B virus-positive hepatocellular carcinoma. Cancer. 2009;115(20):4753–65.
Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002;36(3):692–701.
Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–302.
Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–13.
Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2019.
Portolani N, Baiocchi GL, Molfino S, Benetti A, Gheza F, Giulini SM. Microvascular infiltration has limited clinical value for treatment and prognosis in hepatocellular carcinoma. World J Surg. 2014;38(7):1769–76.
Huang C, Zhu XD, Ji Y, Ding GY, Shi GM, Shen YH, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer. 2017;17(1):58.
Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–64.
Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412 (Epub 2018/02/08).
Sugino T, Yamaguchi T, Hoshi N, Kusakabe T, Ogura G, Goodison S, et al. Sinusoidal tumor angiogenesis is a key component in hepatocellular carcinoma metastasis. Clin Exp Metastasis. 2008;25(7):835–41.
Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62(2):452–65.
Fang JH, Xu L, Shang LR, Pan CZ, Ding J, Tang YQ, et al. Vessels that encapsulate tumor clusters (VETC) pattern is a predictor of sorafenib benefit in patients with hepatocellular carcinoma. Hepatology. 2018.
Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020;71(1):183–95.
Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–5.
Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–39.
Sheng X, Ji Y, Ren GP, Lu CL, Yun JP, Chen LH, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hep Intl. 2020;14(6):1034–47 (Epub 2020/12/29).
Ziol M, Pote N, Amaddeo G, Laurent A, Nault JC, Oberti F, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology. 2018;68(1):103–12.
Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):1002–9.
Kang I, Jang M, Lee JG, Han DH, Joo DJ, Kim KS, et al. Subclassification of Microscopic Vascular Invasion in Hepatocellular Carcinoma. Ann Surg. 2020.
Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47(5):1557–66.
Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7(12):4080–5.
Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117(21):4878–89.
He C, Zhou Z, Jiang H, Yin Z, Meng S, Zhang J, et al. Epithelial-mesenchymal transition is superior to vessels-encapsulate tumor cluster in promoting metastasis of hepatocellular carcinoma: a morphological evidence. J Cancer. 2017;8(1):39–47.
Itoh S, Yoshizumi T, Yugawa K, Imai D, Yoshiya S, Takeishi K, et al. Impact of immune response on outcomes in hepatocellular carcinoma: association with vascular formation. Hepatology. 2020.
Funding
This study was supported by grants from the Clinical Trials Project (5010 Project) of Sun Yat-sen University (No. 5010–2017009), the Clinical Trials Project (308 Project) of Sun Yat-sen University Cancer Center (No. 308–2015-014), the National Natural Science Foundation of China (No. 81871985), and the Natural Science Foundation of Guangdong Province (No. 2017A030310203).
Author information
Authors and Affiliations
Contributions
Acquisition of data: WW, MSC, SHL, CZ, JHW, WSY, and YFZ; Statistical analysi: WW, SHL, CYH, and MSC; Interpretation of results: CYH, CZ, WSY, JHW, and YFZ; Manuscript drafting: LHL, WW, CYH and YHL; Critical revision of the manuscript: LHL, YHL, MSC and RPG; Study concept and design: LHL, YHL, and RPG; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Lianghe Lu, Wei Wei, Chaoyun Huang, Shaohua Li, Chong Zhong, Jiahong Wang, Wushen Yu, Yongfa Zhang, Minshan Chen, Yihong Ling, Rongping Guo declare no potential conflicts of interest.
Ethics approval
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Institutional Review Board and Human Ethics Committees of the five academic hospitals. All patients gave written informed consent for their archived tissue and clinical data to be used for scientific research in this study.
Informed consent
Informed consent was obtained from all patients for inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supporting Figure 1.
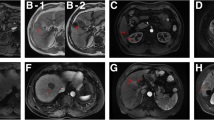
The impacts of VETC and MVI on the long-term outcomes of HCC patients after resection in the entire cohort. VETC: (A) Overall survival; (B) Disease-free survival; (C) Early recurrence; (D) Late recurrence. MVI: (E) Overall survival; (F) Disease-free survival; (G) Early recurrence; (H) Late recurrence. (TIF 1584 kb)
Supporting Figure 2.
The impact of the VETC-MVI model on the long-term outcomes of HCC patients after resection in the SYSUCC cohort: (A) Overall survival. (B) Disease-free survival. (C) Early recurrence. (D) Late recurrence. In addition, the GTFD cohort: (E) Overall survival. (F) Disease-free survival. (G) Early recurrence. (H) Late recurrence. (TIF 2168 kb)
Supporting
Figure 3. Subgroup analysis of the VETC-MVI model on the long-term outcomes of patients after resection in the entire cohort. Tumor size<5 cm: (A) Overall survival; (B) Disease-free survival. Tumor size ≥5 cm: (C) Overall survival; (D) Disease-free survival. Solitary tumor: (E) Overall survival; (F) Disease-free survival. Multiple tumors: (G) Overall survival; (H) Disease-free survival. MTM-positive: (I) Overall survival; (J) Disease-free survival. MTM-negative: (K) Overall survival; (L) Disease-free survival. (TIF 1811 kb)
S
upporting Figure 4. The prognostic value of integrating the VETC and MVI grading systems. MVI grading system: (A) Overall survival; (B) Disease-free survival. Combination of VETC and MVI grading system: (C) Overall survival; (B) Disease-free survival. (TIF 638 kb)
Rights and permissions
About this article
Cite this article
Lu, L., Wei, W., Huang, C. et al. A new horizon in risk stratification of hepatocellular carcinoma by integrating vessels that encapsulate tumor clusters and microvascular invasion. Hepatol Int 15, 651–662 (2021). https://doi.org/10.1007/s12072-021-10183-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10183-w