Abstract
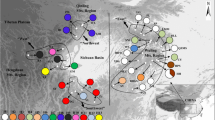
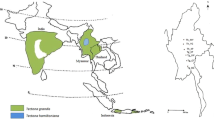
Geographic patterns of genetic variation at chloroplast markers have been successfully used to address the phylogeography and the demographic history of many plant species. Very few studies have however been conducted in important tropical centers of plant biodiversity like the African rainforests. The phylogeography of a tree species widespread in Central African mature forests, Greenwayodendron suaveolens subsp. suaveolens (Annonaceae), was investigated in the Lower Guinea phytogeographic domain (essentially Gabon and Cameroon) by sequencing an intergenic spacer of the chloroplast genome (trnC-petN1R). A total of 11 polymorphic sites, including nine single nucleotide polymorphisms (SNPs), two insertions/deletions and two inversions, defined 12 haplotypes. The taxon is represented by two sympatric varieties (var. suaveolens and var. gabonica) that carried distinct and relatively divergent haplotypes. These varieties, also well distinguishable morphologically, might therefore represent true biological species. The variety suaveolens, more common and more widespread than the variety gabonica, was represented by ten haplotypes. This taxon showed a weak but statistically significant phylogeographic structure, indicating that two sets of related haplotypes essentially occurred respectively in the northern and the southern hemispheres. These results suggest that the distribution of Greenwayodendron suaveolens subsp. suaveolens, which is currently continuous in the Lower Guinea domain, might have been more fragmented in the past, possibly in relation with Pleistocene forest fragmentation.



Similar content being viewed by others
References
Anthony NM, Johnson-Bawe M, Jeffery K et al (2007) The role of Pleistocene refugia and rivers in shaping gorilla genetic diversity in central Africa. Proc Natl Acad Sci U S A 104(51):20432–20436
Aubréville A (1959) La flore forestière de la Côte d’Ivoire. Centre Technique Forestier Tropical, Paris
Avise JC (2009) Phylogeography: retrospect and prospect. J Biogeogr 36(1):3–15
Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16(1):37–48
Betti JL (2004) An ethnobotanical study of medicinal plants among the Baka pygmies in the Dja biosphere reserve, Cameroon. Afr Study Monogr 25(1):1–27
Birky CW (1995) Uniparental inheritance of mitochondrial and chloroplast genes—mechanisms and evolution. Proc Natl Acad Sci U S A 92(25):11331–11338
Blake S, Deem SL, Mossimbo E et al (2009) Forest elephants: tree planters of the Congo. Biotropica 41(4):459–468
Born C (2007) Diversité génétique et dynamique des forêts d’Afrique centrale: une étude multi-échelle de la structure de la diversité génétique d’un arbre pionnier Aucoume klaineana. Université Montpellier II, Montpellier
Bouquet A (1969) Féticheurs et Médecines traditionelles du Congo (Brazzaville). ORSTOM, Paris
Brouat C, Gielly L, McKey D (2001) Phylogenetic relationships in the genus Leonardoxa (Leguminosae: Caesalpinioideae) inferred from chloroplast trnL intron and trnL-trnF intergenic spacer sequences. Am J Bot 88(1):143–149
Cannon CH, Manos PS (2003) Phylogeography of the Southeast Asian stone oaks (Lithocarpus). J Biogeogr 30(2):211–226
Cavender-Bares J, Kozak KH, Fine PVA et al (2009) The merging of community ecology and phylogenetic biology. Ecol Lett 12(7):693–715
Dick CW, Bermingham E, Lemes MR et al (2007) Extreme long-distance dispersal of the lowland tropical rainforest tree Ceiba pentandra L. (Malvaceae) in Africa and the Neotropics. Mol Ecol 16(14):3039–3049
Droissart V (2009) Etude taxonomique et biogéographique des plantes endémiques d’Afrique centrale atlantique: le cas des Orchidaceae. Université Libre de Bruxelles, Belgique
Dubost G (1983) Le comportement de Cephalophus monticola Thunberg et C.dorsalis Gray et la place de céphalophes au sein des ruminants (2ième partie). Mammalia 47(3):282–303
Duminil J, Caron H, Scotti I et al (2006) Blind population genetics survey of tropical rainforest trees. Mol Ecol 15(12):3505–3513
Duplantier JM, Orsini P, Thohari M et al (1984) Echantillonnage des populations de Muridés: influence du protocole de piégeage sur l’estimation des paramètres démographiques. Mammalia 48(1):129–141
Dutech C, Maggia L, Joly HI (2000) Chloroplast diversity in Vouacapoua americana (Caesalpiniaceae), a neotropical forest tree. Mol Ecol 9(9):1427–1432
Dutech C, Maggia L, Tardy C et al (2003) Tracking a genetic signal of extinction-recolonization events in a neotropical tree species: Vouacapoua americana aublet in french guiana. Evolution 57(12):2753–2764
Engler A, Diels L (1901) Annonaceae. Monogr Afrik Pflanzen-Fam Gatt VI:41–43
Feer F (1995) Morphology of fruits dispersed by African forest elephants. Afr J Ecol 33(3):279–284
Gautier-Hion A, Michaloud G (1989) Are figs always keystone resources for tropical frugivorous vertebrates? A test in Gabon. Ecology 70(6):1826–1833
Grivet D, Petit RJ (2003) Chloroplast DNA phylogeography of the hornbeam in Europe: evidence for a bottleneck at the outset of postglacial colonization. Conserv Genet 4(1):47–56
Haffer J (1969) Speciation in Amazon rainforest birds. Science 165:131–137
Hardy OJ, Vekemans X (2002) SPAGEDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2(4):618–620
Heuertz M, Carnevale S, Fineschi S et al (2006) Chloroplast DNA phylogeography of European ashes, Fraxinus sp (Oleaceae): roles of hybridization and life history traits. Mol Ecol 15(8):2131–2140
Hewitt GM (2004) Genetic consequences of climatic oscillations in the quaternary. Philos Trans R Soc Lond Ser B-Biol Sci 359(1442):183–195
Holbrook KM, Smith TB, Hardesty BD (2002) Implications of long-distance movements of frugivorous rain forest hornbills. Ecography 25(6):745–749
Kress WJ, Wurdack KJ, Zimmer EA et al (2005) Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci U S A 102(23):8369–8374
Lamidi M, DiGiorgio C, Delmas F et al (2005) In vitro cytotoxic, antileishmanial and antifungal activities of ethnopharmacologically selected Gabonese plants. J Ethnopharmacol 102(2):185–190
Le Thomas A (1965) Notes sur quelques Annonaceae Africaines. Adansonia V(3):443–454
Le Thomas A (1969) Flore du Gabon vol 16, Annonacées. Muséum National d’Histoire Naturelle, Paris
Lowe AJ, Gillies ACM, Wilson J et al (2000) Conservation genetics of bush mango from central/west Africa: implications from random amplified polymorphic DNA analysis. Mol Ecol 9(7):831–841
Lowe AJ, Harris D, Dormontt E et al (2010) Testing putative African tropical forest refugia using chloroplast and nuclear DNA phylogeography. Tropical Plant Biol 3(1):50–62
Maley J (1996) The African rain forest—main characteristics of changes in vegetation and climate from the upper cretaceous to the quaternary. Proc R Soc Edinb 104B:31–73
Mols JB, Gravendeel B, Chatrou LW et al (2004) Identifying clades in Asian Annonaceae: monophyletic genera in the polyphyletic Miliuseae. Am J Bot 91(4):590–600
Muloko-Ntoutoume N, Petit RJ, White L et al (2000) Chloroplast DNA variation in a rainforest tree (Aucoumea klaineana, Burseraceae) in Gabon. Mol Ecol 9(3):359–363
Nicolas V, Mboumba JF, Verheyen E et al (2008) Phylogeographic structure and regional history of Lemniscomys striatus (Rodentia: Muridae) in tropical Africa. J Biogeogr 35(11):2074–2089
Parmentier I, Malhi Y, Senterre B et al (2007) The odd man out? Might climate explain the lower tree alpha-diversity of African rain forests relative to Amazonian rain forests? J Ecol 95(5):1058–1071
Petit RJ, Brewer S, Bordacs S et al (2002) Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manage 156(1–3):49–74
Petit RJ, Aguinagalde I, de Beaulieu JL et al (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300(5625):1563–1565
Plana V (2004) Mechanisms and tempo of evolution in the African Guineo–Congolian rainforest. Philos Trans R Soc Lond Ser B Biol Sci 359(1450):1585–1594
Plana V, Gascoigne A, Forrest LL et al (2004) Pleistocene and pre-pleistocene Begonia speciation in Africa. Mol Phylogenet Evol 31(2):449–461
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144(3):1237–1245
Querouil S, Verheyen E, Dillen M et al (2003) Patterns of diversification in two African forest shrews: Sylvisorex johnstoni and Sylvisorex ollula (Soricidae, Insectivora) in relation to paleo-environmental changes. Mol Phylogenet Evol 28(1):24–37
Raponda-Walker A, Sillans R (1961) Les plantes utiles du Gabon. Fondation Raponda-Walker, Sepia
Robbrecht E (1996) Geography of African Rubiaceae with reference to glacial rain forest refuges. In: van der Maesen LJG, van der Burgt XM, van Medenbach de Rooy JM (eds) The biodiversity of African plants: proceedings XIVth AETFAT congress. Kluwer, Netherlands
Silberbauer-Gottsberger I, Gottsberger G, Webber AC (2003) Morphological and functional flower characteristics of New and Old World Annonaceae with respect to their mode of pollination. Taxon 52(4):701–718
Sosef M (1994) Refuge begonias: taxonomy, phylogeny and historical biogeography of Begonia sect. Loasibegonia and sect. Scutobegonia in relation to glacial rain forest refuges in Africa. Agric Univ Wageningen Pap 94(1):1–306
Sosef M, Issembe YA, Bouroubou Bourbou HP et al (2004) Botanical diversity of the pleistocene forest refuge Monts Doudou. Mem Calif Acad Sci 28:17–91
Sosef MSM, Wieringa JJ, Jongkind CCH et al (2006) Check-list des plantes vasculaires du Gabon/Checklist of Gabonese vascular plants. Scr Bot Belg 35:1–438
Stévart T (2003) Etude taxonomique, écologique et phytogéographique des Orchidaceae en Afrique centrale atlantique. Université Libre de Bruxelles, Belgique
Stocks G, Seales L, Paniagua F et al (2008) The geographical and institutional distribution of ecological research in the tropics. Biotropica 40(4):397–404
Telfer PT, Souquiere S, Clifford SL et al (2003) Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx. Mol Ecol 12(7):2019–2024
Verdcourt B (1969) The status of the genus Polyalthia Blume (Annonaceae) in Africa. Adansonia ser 2 9(1):87–94
Verdcourt B (1971) Annonaceae. In: Milne-Redhead E, Polhill RM (eds) Flora of tropical East Africa. Royal Botanic Gardens, Kew
White F (1979) The Guineo–Congolian region and its relationships to other phytochoria. Bull Jard Bot Nat Belg 49:11–55
Whitney KD, Fogiel MK, Lamperti AM et al (1998) Seed dispersal by Ceratogymna hornbills in the Dja Reserve, Cameroon. J Trop Ecol 14:351–371
Yoo HD, Cremin PA, Zeng L et al (2005) Suaveolindole, a new mass-limited antibacterial indolosesquiterpene from Greenwayodendron suaveolens obtained via high-throughput natural products chemistry methods. J Nat Prod 68(1):122–124
Acknowledgments
GD is a PhD candidate funded by the Belgian Fund for Training to Research in Industry and Agriculture (FRIA). JD and OJH are employed by the National Fund for Scientific Research of Belgium (FNRS), respectively as Postdoctoral Researcher and Research Associate. JD was also funded by the Gembloux Agricultural University (FUSAGx, Belgium) via the project PPR 10.000. MH acknowledges a Postdoctoral Researcher position of the FNRS and a scientific visit to the Royal Botanic Gardens, Kew, funded by the EU Synthesys programme (GB-TAF-1305). GD’s field work was financed by FNRS, Communauté française de Belgique and Cassel Fund. Financial and logistic support in Gabon was provided by the Central African Program of the Missouri Botanical Garden and the Wildlife Conservation Society through the Central Africa Regional Program of the Environment (CARPE). This work was funded by the Fonds de la Recherche Collective FRFC-FNRS project 2.4.576.07 and by the IFORA (îles forestières africaines) project, financed by the French ANR (Agence Nationale de la Recherche) under the ANR-BIODIV program. We would like to acknowledge the Missouri Botanical Garden (Central African Program), the CENAREST (Gabon), the Smithsonian Institution (Gabon Biodiversity Program), Prof. Bonaventure Sonké (Université de Yaoundé I, Cameroon) and Prof. Charles Doumenge (CIRAD) for facilitating field work and sample collection. We thank Daniel Geerinck for help in taxonomic issues.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Christopher Dick
DNA sequences presented in this paper have been deposited with the Genebank/EMBL libraries under accession numbers: GU121907-GU121918
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
Sampled populations of Greenwayodendron suaveolens and their cpDNA haplotypes composition. Geographic coordinates are given in decimal degrees (minus sign for southern hemisphere). (DOC 82 kb)
Rights and permissions
About this article
Cite this article
Dauby, G., Duminil, J., Heuertz, M. et al. Chloroplast DNA Polymorphism and Phylogeography of a Central African Tree Species Widespread in Mature Rainforests: Greenwayodendron suaveolens (Annonaceae). Tropical Plant Biol. 3, 4–13 (2010). https://doi.org/10.1007/s12042-010-9041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-010-9041-6