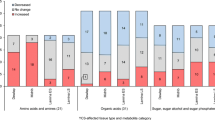
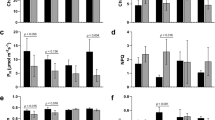
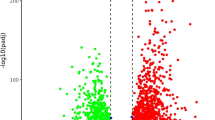
Abstract
In C4 sugarcane (Saccharum spp. hybrids), photosynthetic activity has been shown to be regulated by the demand for carbon from sink tissues. There is evidence, from other plant species, that sink-limitation of photosynthesis is facilitated by sugar-signaling mechanisms in the leaf that affect photosynthesis through regulation of gene expression. In this work, we manipulated leaf sugar levels by cold-girdling leaves (5°C) for 80 h to examine the mechanisms whereby leaf sugar accumulation affects photosynthetic activity and assess whether signaling mechanisms reported for other species operate in sugarcane. During this time, sucrose and hexose concentrations above the girdle increased by 77% and 81%, respectively. Conversely, leaf photosynthetic activity (A) and electron transport rates (ETR) decreased by 66% and 54%, respectively. Quantitative expression profiling by means of an Affymetrix GeneChip Sugarcane Genome Array was used to identify genes responsive to cold-girdling (56 h). A number of genes (74) involved in primary and secondary metabolic pathways were identified as being differentially expressed. Decreased expression of genes related to photosynthesis and increased expression of genes involved in assimilate partitioning, cell wall synthesis, phosphate metabolism and stress were observed. Furthermore four probe sets homologous to trehalose 6-phosphate phosphatase (TPP; EC 5.3.1.1) and trehalose 6-phosphate synthase (TPS; EC 2.4.1.15) were up- and down-regulated, respectively, indicating a possible role for trehalose 6-phosphate (T6P) as a putative sugar-sensor in sugarcane leaves.




Similar content being viewed by others
References
Allison JCS, Williams HT, Pammenter NW (1997) Effect of specific leaf nitrogen on photosynthesis of sugarcane. Ann Appl Biol 63:135–144
Amaya A, Cock JH, Hernandez A, Irvine J (1995) Bioligía. In: Casselett C, Torres J, Isaacs C (eds) El cultivo de la Caňa en la zona azucarera de Colombia. Cenicaňa, Cali, Colombia, pp 31–62
Altschul SF, Madden TL, Schaffer AA, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arruda P (2001) Sugarcane transcriptome. A landmark in plant genomics in the tropics. Genet Mol Biol 24:1–4
Basu PS, Sharma A, Garg ID, Sukumaran NP (1999) Tuber sink modifies photosynthetic response in potato under water stress. Environ Exp Bot 42:25–29
Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. The Plant Cell 17:3257–3281
Bugos RC, Chiang VL, Zhang XH, Campbell ER, Podila GK, Campbell WH (1995) RNA isolation from plant tissues recalcitrant to extraction in guanidine. Biotechniques 19:734–737
Bull TA, Tovey DA (1974) Aspects of modelling sugar cane growth by computer simulation. Proc Int Soc Sugarcane Technol 165:1021–1032
Carson DL, Huckett BI, Botha FC (2002) Sugarcane ESTs differentially expressed in immature and maturing internodal tissue. Plant Sci 162:289–300
Casu RE, Dimmock CM, Chapman SC, Grof CPL, McIntyre CL, Bonnett GD, Manners JM (2004) Identification of differentially expressed transcripts from maturing stem of sugarcane by in silico analysis of stem expressed sequence tags and gene expression profiling. Plant Mol Biol 54:503–517
Casu RE, Grof CPL, Rae AL, McIntyre CL, Dimmock CM, Manners JM (2003) Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues in sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol 52:371–386
Casu RE, Jarmey J, Bonnett G, Manners J (2007) Identification of transcripts associated with cell wall metabolism and development in the stem of sugarcane by Affymetrix GeneChip Sugarcane Genome Array expression profiling. Funct Integr Genomics 7:153–167
Ciereszko I, Johnsson H, Hurry V, Kleczkowski LA (2001) Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta 212:598–605
Davies C, Robinson SP (2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response genes. Plant Physiol 122:803–812
Du YC, Nose A, Kondo A, Wasano K (2000) Diurnal changes in photosynthesis in sugarcane leaves. II. Enzyme activities and metabolite levels relating to sucrose and starch metabolism. Plant Prod Sci 3:9–16
Eastmond PJ, Li Y, Graham IA (2003) Is trehalose-6-phosphate a regulator of sugar metabolism in plants? J Exp Bot 54:533–537
Eastmond PJ, van Dijken AJ, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JD, Smeekens SC, Graham IA (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29:225–235
Edwards GE, Baker NR (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37:89–102
Ehness R, Ecker M, Godt DE, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9:1825–1841
Franck N, Vaast P, Genard M, Dauzat J (2006) Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiol 26:517–525
Franco-Zorrilla JM, Martin AC, Leyva A, Paz-Ares J (2005) Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol 138:847–857
Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M (2006) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16:3304–3325
Grof C, Campbell J (2001) Sugarcane sucrose metabolism: scope for molecular manipulation. Aust J Plant Physiol 28:1–12
Goldschmidt EE, Huber SC (2001) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99:1443–1448
Gutiérrez-Miceli FA, Morales-Torres R, de Jesús Espinosa-Castañeda Y, Rincón-Rosales R, Mentes-Molina J, Oliva-Llaven MA, Dendooven L (2004) Effects of partial defoliation on sucrose accumulation, enzyme activity and agronomic parameters in sugar cane (Saccharum spp.). J Agron Crop Sci 190:256–261
Hartt CE, Burr GO (1967) Factors affecting photosynthesis in sugarcane. Proc Int Soc Sugarcane Technol 12:590–609
Huckett BA, Botha FC (1995) Stability and potential use of RAPD markers in a sugarcane genealogy. Euphytica 86:117–125
Iglesias DJ, Lliso I, Tadeo FR, Talon M (2002) Regulation of photosynthesis through source: sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol Plant 116:563–572
Ingelbrecht IL, Mandelbaum CI, Mirkov TE (1998) Highly sensitive northern hybridization using a rapid protocol for downward alkaline blotting of RNA. BioTechniques 25:420–425
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acid Res 4(e15):1–8
Jackson PA (2005) Breeding for improved sugar content in sugarcane. Field Crops Res 92:277–290
Jones MG, Outlaw WH, Lowry OH (1977) Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol 60:379–383
Kalt-Torres W, Kerr PS, Usuda H, Huber SC (1987) Diurnal changes in maize leaf photosynthesis. Plant Physiol 83:283–288
Krapp A, Hofman B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: mechanism for the sink regulation of photosynthesis? Plant J 3:817–828
Krapp A, Quick WP, Stitt W (1991) Ribulose-1,5-bisphosphate carboxylase-oxygenase, other Calvin cycle enzymes and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transcription stream. Planta 186:58–59
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady state transcript levels after cold-girdling leaves. Planta 195:313–323
Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P (2005) Trehalose 6-phosphate regulates starch synthesis via post-translational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 102:11118–11123
Lawlor DW (1987) Photosynthesis: metabolism, control and physiology. Longman, Harlow, UK
Lee JM, Williams ME, Tingey SV, Rafalski AJ (2002) DNA array profiling of gene expression changes during maize embryo development. Funct Integr Genomics 2:13–27
Leibbrandt NB, Snyman SJ (2003) Stability of gene expression and agronomic performance of a transgenic herbicide-resistant sugarcane line in South Africa. Crop Sci 43:671–677
Lloyd JC, Zakhleniuk OV (2004) Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot 55:1221–1230
Lunn JE, Furbank RT (1999) Sucrose biosynthesis in C4 plants. New Phytol 143:221–237
Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397:139–148
Ma H, Albert HA, Paull R, Moore PH (2000) Metabolic engineering of invertase activities in different subcellular compartments affects sucrose accumulation in sugarcane. Aust J Plant Physiol 27:1021–1030
Masclaux-Daubresse C, Purdy S, Lemaitre T, Pourtau N, Taconnat L, Renou JP, Wingler A (2007) Genetic variation suggests interaction between cold acclimation and metabolic regulation of leaf senescence. Plant Physiol 143:434–446
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171:759–770
McCormick AJ, Cramer MD, Watt DA (2008) Changes in photosynthetic rates and gene expression of leaves during a source–sink perturbation in sugarcane. Ann Bot 101:89–102
McCormick AJ, Cramer MD, Watt DA (2008) Regulation of photosynthesis by sugars in sugarcane leaves. J Plant Physiol. doi:10.1016/j.jplph.2008.01.008
Minchin PEH, Thorpe MR, Farrar JF, Koroleva OA (2002) Source–sink coupling in young barley plants and control of phloem loading. J Exp Bot 53:1671–1676
Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH (2007) Genome-wide analysis of the Arabidopsis transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol 143:156–171
Nielsen TH, Krapp A, Röber-Schwarz U, Stitt M (1998) The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ 21:443–454
Paul MJ (2007) Trehalose 6-phosphate. Curr Opin Plant Biol 10:303–309
Paul MJ, Driscoll SP (1997) Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ 20:110–116
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1381–1400
Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54:539–547
Paul MJ, Pellny TK, Goddijn IJM (2001) Enhancing photosynthesis with sugar signals. Trends Plant Sci 6:197–200
Pego JV, Kortsee AJ, Huijser C, Smeekens SCM (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51:407–416
Pellny TK, Ghannoum O, Conroy JP, Schluepmann H, Smeekens S, Andralojc J, Krause KP, Goddijn O, Paul MJ (2004) Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnol 2:71–82
Pieters AJ, Paul MJ, Lawlor DW (2001) Low sink demand limits photosynthesis under Pi deficiency. J Exp Bot 52:1083–1091
Prioul JL, Reyss A (1988) Rapid variations in the content of the RNA of the small subunit of ribulose-1,5-bisphosphate carboxylase of mature tobacco leaves in response to localized changes in light quantity. Relationships between the activity and quantity of the enzyme. Planta 174:488–494
Ramon M, Rolland F (2007) Plant development: introducing trehalose metabolism. Trends Plant Sci 12:185–188
Rodermel S, Haley J, Jiang CZ, Tsai CH, Bogorad L (1996) A mechanism for intergenomic integration: abundance of ribulose bisphosphate carboxylase small-subunit protein influences the translation of the large-subunit mRNA. Proc Natl Acad Sci USA 93:3881–3885
Roitsch T, Balibrea ME, Hofmann M, Proeis R, Sinna AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54:513–524
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14:185–205
Sawers RJH, Liu P, Anufrikova K, Hwang JT, Brutnell TP (2007) A multi-treatment experimental system to examine photosynthetic differentiation in the maize leaf. BMC Genomics 8:1–13
Schäfer C, Simper H, Hofmann B (1992) Glucose feeding results in co-ordinated changes of chlorophyll content, ribulose-1,5-bisphosphate carboxylase/oxygenase activity and photosynthetic potential in photoautotrophic suspension-cultured cells of Chenopodium rubrum. Plant Cell Environ 15:343–350
Schluepmann H, van Dijken A, Smeekens S, Paul M (2003) Trehalose-6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:6849–6854
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51:49–81
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 147:637–646
Stitt M, Quick WP (1989) Photosynthetic carbon partitioning: its regulation and possibilities for manipulation. Physiol Plant 77:633–641
Stitt M, Gibon Y, Lunn JE, Piques M (2007) Multilevel genomics analysis of carbon signalling during low carbon availability: coordinating the supply and utilization of carbon in a fluctuating environment. Funct Plant Biol 34:526–549
Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P (2003) Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J 35:490–500
Toroser D, Plaut Z, Huber SC (2000) Regulation of a plant SNF1-related protein kinase by glucose-6-phosphate. Plant Physiol 123:403–411
Van Oosten JJ, Besford RT (1994) Sugar feeding mimics effect of acclimation to high CO2: rapid downregulation of RuBisCO small subunit transcripts, but not of the large subunit transcripts. J Plant Physiol 143:306–312
Watt DA, McCormick AJ, Govender C, Carson DL, Cramer MD, Huckett BI, Botha FC (2005) Increasing the utility of genomics in unraveling sucrose accumulation. Field Crops Res 92:149–158
Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci 5:283–290
Wu L, Birch RG (2007) Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotechnol J 5:109–117
Acknowledgments
The authors are grateful for funding provided by the South African Sugarcane Research Institute, SA Sugar Association Trust Fund for Education and the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dr. Paul Moore
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
List of probe sets differentially expressed during the cold-girdling treatment. Putative identity was assigned using the BLASTX function within the National Centre of Biotechnological Information (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov). Where E values are absent, probe sets homology was matched to those assigned by Casu et al. [12]. Fold changes indicate statistical significance values (P < 0.05) as determined by ANOVA (n = 4) (XLS 128 KB)
Fig. 1
Normalised gene expression profile comparison between cold-girdled (56 h) and control leaves. Up- and down-regulation of genes in controls (n = 4) is seen in red and blue, respectively, whereas the reverse applies for the cold-girdled leaves. Genes that remain unaffected by the treatment are depicted in yellow (GIF 2.24 MB)
Supplementary Fig. 1
High resolution image file (TIFF 495 kb)
Rights and permissions
About this article
Cite this article
McCormick, A.J., Cramer, M.D. & Watt, D.A. Differential Expression of Genes in the Leaves of Sugarcane in Response to Sugar Accumulation. Tropical Plant Biol. 1, 142–158 (2008). https://doi.org/10.1007/s12042-008-9013-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-008-9013-2