Abstract
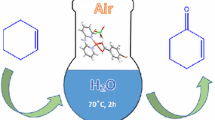
An environmentally friendly protocol is described for an economic, practical laboratory-scale oxidation of primary and secondary alcohols to aldehydes and ketones, using a bis-chloro-bridged binuclear Cu(II) complex [(HL)Cu(μ 2-Cl)2Cu(HL)]*1.5 CH3OH as catalyst. The catalyst was prepared in situ from commercially available reagents and is characterized by single crystal X-ray analysis, FT-IR, UV-visible spectra, mass spectrometry, and powder x-ray diffraction (PXRD). The geometry of the complex has been optimized using the B3LYP level of theory confirming the experimental data. Our results demonstrated well the efficiency, selectivity and stability of this new catalyst in the oxidation of alcohols in ethanol and tert-butyl hydroperoxide (tBuOOH) as a green solvent and oxidant, respectively. Turnover number and reusability have proven the high efficiency and relative stability of the catalyst.

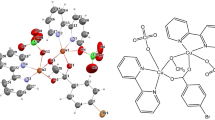
A novel [(HL)Cu(μ2-Cl)2Cu(HL)]*1.5CH3OH complex was used as an efficient catalyst for the oxidation of alcohols. It was synthesized, using (E)-1-(((2-hydroxypropyl)imino)methyl)naphthalen-2-ol as a monoanionic and tridentate Schiff base ligand, and characterized by IR and UV-Visible spectroscopy and single-crystal X-ray analysis. The geometry of the complex was optimized using the B3LYP level of theory.





Similar content being viewed by others
References
Busacca C A, Fandrick D R, Song J J and Senanayake C H 2011 Adv. Synth. Catal. 353 1825
Arends W C E and Sheldon R A 2010 In Modern Oxidation Methods 2nd ed. J E Bäckvall (ed.) (Weinheim: Wiley–VCH) pp. 147–185
Hoover J M, Steves J E and Stahl S S 2012 Nature Protocols 7 1161
Fatiadi A J 1976 Synthesis 65
Taylor R J K, Reid M, Foot J and Raw S A 2005 Acc. Chem. Res. 38 851
Pfitzner K E and Moffatt J G 1963 J. Am. Chem. Soc. 85 3027
Mancuso A J, Brownfain D S and Swern D 1979 J. Org. Chem. 44 4148
Tidwell T T 1990 Synthesis 857
Moreno A L, Tejeda D C, Calbo J, Naeimi A, Bermejo F A, Ortí E and Pérez E M 2014 Chem. Commun. 50 9372
Aguiló J, Naeimi A, Bofill R, Bunz H M, Llobet A, Escriche L, Sala X and Albrech M 2014 New J. Chem. 38 1980
Rezaeifard A, Jafarpour M, Naeimi A and Haddad R 2012 Green Chem. 14 3386
Rezaeifard A, Jafarpour M, Naeimi A and Salimi M 2012 Inorg. Chem. Commun. 15 230
Rezaeifard A, Jafarpour M and Naeimi A 2011 Catal. Commun. 16 240
Rezaeifard A, Jafarpour M, Naeimi A and Mohammadi K 2012 J. Mol. Catal. A: Chem. 357 141
Rezaeifard A, Jafarpour M, Naeimi A and Kaafi S 2011 Catal. Commun. 12 761
Bruker, 1999 SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA
Burla M C, Caliandro R, Camalli M, Carrozzini B, Cascarano G L, De Caro L, Giacovazzo C, Polidori G and Spagna R 2005 J. Appl. Crystallogr. 38 381
Sheldrick G M 1997 SHELXL97. Program for crystal structure refinement. University of Gottingen, Germany
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr , Vreven T., Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A 2004 Gaussian 03, Revision E.01 (Gaussian, Inc.: Wallingford, CT)
Biegler-Konig F., AIM 2000 version 1.0, 1998 University of Applied. Science, Bielefeld, Germany
Tarafder M, Kasbollah A, Crouse K, Ali A, Yamin B and Fun H K 2001 Polyhedron 20 2363
Vafazadeh R, Khaledi B, Willis A C and Namazian M 2011 Polyhedron 30 1815
Trzesowska-Kruszynska A 2012 J. Mol. Struc. 1017 72
Thakurta S, Roy P, Rosair G, Gómez-García C J, Garribba E and Mitra S 2009 Polyhedron 28 695
Hathaway B, Wilkinson G, Gillard R and McCleverty J 1987 In Comprehensive Coordination Chemistry Vol. 5 (Oxford: Pergamon)
Addison A W, Rao T N, Reedijk J, van Rijn J and Verschoor G C 1984 J. Chem. Soc., Dalton Trans. 1349
Bader R F 1991 Chem. Rev. 91 893
Bader R F 1990 In Atoms in Molecules: A Quantum Theory (London: Oxford University Press)
Raissi H, Yoosefian M, Mollania F and Khoshkhou S 2013 Struct. Chem. 24 123
Raissi H, Yoosefian M and Mollania F 2012 Int. J. Quantum Chem. 112 2782
Fazli M, Jalbout A, Raissi H, Ghiassi H and Yoosefian M 2009 J. Theor. Comput. Chem. 8 713
Yoosefian M, Barzgari Z and Yoosefian J 2014 Struct. Chem. 25 9
Raissi H, Khanmohammadi A, Yoosefian M and Mollania F 2013 Struct. Chem. 24 1121
Raissi H, Yoosefian M, Mollania F, Farzad F and Nowroozi A R 2011 Comput. Theor. Chem. 966 299
Meunier B 1992 Chem. Rev. 92 1411
McGarrigle E M and Gilheany D G 2005 Chem. Rev. 105 1563
Acknowledgements
We are thankful to University of Jiroft and Vali-e-Asr University of Rafsanjan Research Council for their support on this work. We also thank Dr. Emilio Perez for his valuable comments to improve our project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Figures S1 and S2 and tables S1 and S2 are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
NAEIMI, A., SAEEDNIA, S., YOOSEFIAN, M. et al. A novel dinuclear schiff base copper complex as an efficient and cost effective catalyst for oxidation of alcohol: Synthesis, crystal structure and theoretical studies. J Chem Sci 127, 1321–1328 (2015). https://doi.org/10.1007/s12039-015-0896-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0896-9