Abstract
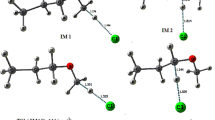
The present study deals with the decomposition of haloalkoxy radical (CH3CFClO) formed from 1,1-dichloro-1-fluoroethane (HCFC-141b) in the atmosphere. The study is performed using ab-initio quantum mechanical methods. Out of the three plausible pathways of decomposition of the titled species, the one that involved the C–C bond scission and the other occurring via Cl-atom elimination have been considered for detailed study. The geometries of the reactant, products and transition states involved in the decomposition pathways are optimized and characterized at MP2 level of theory using 6-311G(d,p) basis set. Single point energy calculations have been performed at G2(MP2) level of theory. The path involving the Cl-elimination is found to be dominant and found to occur with a barrier height of 3.6 kcal mol − 1 whereas the C–C bond scission path proceeds with a barrier of 10.0 kcal mol − 1. The thermal rate constants for the above two decomposition pathways are evaluated using Canonical Transition State Theory (CTST) and these are found to be 2.9 × 108 s − 1 and 4.3 × 105 s − 1 for Cl-elimination and C–C bond scission respectively at 298 K and 1 atm. pressure. The existence of transition states on the corresponding potential energy surface is ascertained by the occurrence of only one imaginary frequency obtained during the frequency calculation. The Intrinsic Reaction Coordinate (IRC) calculation has also been performed to confirm the smooth connection of the TS to the reactant and the products.

Computational studies using G2(MP2) methods have been performed to investigate the decompositions channels of CH3CFClO radical formed from HCFC-141b in the atmosphere. The results show that Cl elimination pathway is the dominant one. Rate constants of different channels considered have also been evaluated using Canonical Transition State Theory (CTST).
Similar content being viewed by others
References
Solomon S 1990 Nature 347 6291
Molina M J and Rowland F S 1974 Nature 249 810
Rowland F S and Molina M J 1994 Chem. Eng. News. 8 72
Weubbles D J 1983 J. Geophys. Res. 88 1433
Scientific assessment of stratospheric ozone, 1989, Vol II AFEAS Report, World Meteorological Organization, Global Ozone Research and Monitoring Project, Report No-20
Wayne R P 2001 The chemistry of atmospheres (Oxford: Clarendon Press)
Ravishankara A R and Lovejoy E R 1994 J. Chem. Soc. Faraday Trans. 90 2159
Jonathan S N and Stephanle R S 1992 Environ. Sci. Tech. 26 739
AFEAS (Alternative Fluorocarbons Environmental Acceptability Study) 1997 Production, Sales and Atmospheric Release of Fluorocarbons through 1995 (Washington, DC: AFEAS Program Office)
Shirai T and Makide Y 1998 Chem. Lett. 4 357
Oram D E, Reeves C E, Penkett S A and Fraser P J 1995 Geophys. Res. Lett. 22 2741
Lee J M, Sturges W T, Penkett S A, Oram D E, Schmidt U, Engel A and Bauer R 1995 Geophys. Res. Lett. 22 1369
Wu Fuxiang and Carr R W 1995 J. Phys. Chem. 99 3128
DeMore W B, Sander S P, Howard C J, Ravishankara A R, Golden D M, Kolb C E, Hampson R F, Molina M J and Kurylo M J 1992 Chemical kinetics and photochemical data for use in stratospheric modeling, Evaluation Number 10, JPL Publication 92–20
Wallington T J and Nielsen O J 1991 Int. J. Chem. Kinet. 23 785
Brasseur G P and Orlando J J (ed) 1999 Atmospheric chemistry and global change (New York: Oxford University Press)
Wallington T J, Hurley M D, Francheboud J M, Orlando J J, Tyndall G S, Sehested J, Møgelberg T E and Nielsen O J 1996 J. Phys. Chem. 100 18116
Zellner R (ed) 1999 Global aspect of atmospheric chemistry (Darmstadt: SteinKopff)
Hehre W J, Radom L, Schleyer P V R and Pople J A 1986 Ab initio Molecular orbital theory (New York: Wiley)
Frisch M J et al 2004 Gaussian 03 (Revision C.02) (Wallingford CT: Gaussian Inc)
Moller C and Plesset M S 1934 Phys. Rev. 46 618
Hariharan P C and Pople J A 1973 Theo. Chem. Act. 28 213
Gonzalez C and Schlegel H B 1990 J. Phys. Chem. 94 5523
Curtiss L A, Raghavachari K and Pople J A 1993 J. Chem. Phys. 98 1293
Curtiss L A, Redfern P C, Smith B J and Radom Leo 1996 J. Chem. Phys. 104 5148
Frisch A, Nielsen A B and Holder A J 2003 GaussView Users Manual (Gaussian Inc, PA, USA)
Hou H, Wang B and Gu Y 2000 J. Phys. Chem. A 104 1570
Scott A P and Radom L 1996 J. Phys. Chem. 100 16502
Bhatnagar A and Carr R W 1995 J. Phys. Chem. 99 17573
Truhlar D G, Garrett B C and Klippenstein S J 1996 J. Phys. Chem. 100 12771
Wigner E P 1977 Z. Phys. Chem. 81 2572
Hou H, Wang B and Gu Y 2000 Phys. Chem. Chem. Phys. 2 61
Wallington T J, Hurley M D, Ball J C, Ellermann T, Nielsen O J and Sehested J 1994 J. Phys. Chem. 98 5435
Stevens J E, Khayat R A J, Radkevich O and Brown J 2004 J. Phys. Chem. 108 11354
Caralp F, Devolder P, Fittschen C, Gomez N, Hippler H, Mereau R, Rayez M T, Striebel F and Viskolcz B 1999 Phys. Chem. Chem. Phys. 1 2935
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SINGH, H.J., MISHRA, B.K. Computational study on decomposition kinetics of CH 3 CFClO radical. J Chem Sci 123, 733–741 (2011). https://doi.org/10.1007/s12039-011-0117-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-011-0117-0