Abstract
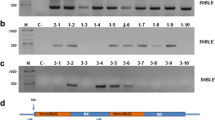
Frankia is a unique actinobacterium having abilities to fix atmospheric dinitrogen and to establish endosymbiosis with trees, but molecular bases underlying these interesting characteristics are poorly understood because of a lack of stable transformation system. Extremely high GC content of Frankia genome (>70%) can be a hindrance to successful transformation. We generated a synthetic gentamicin resistance gene whose codon usage is optimized to Frankia (fgm R) and evaluated its usefulness as a selection marker using a transient transformation system. Success rate of transient transformation and cell growth in selective culture were significantly increased by use of fgm R instead of a native gentamicin resistance gene, suggesting that codon optimization improved translation efficiency of the marker gene and increased antibiotic resistance. Our result shows that similarity in codon usage pattern is an important factor to be taken into account when exogenous transgenes are expressed in Frankia cells.



Similar content being viewed by others
References
Alloisio N, Felix S, Marechal J, Pujic P, Rouy Z, Vallenet D, Medigue C and Normand P 2007 Frankia alni proteome under nitrogen-fixing and nitrogen-replete conditions. Physiol. Plant. 130 440–453
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA and Struhl K 1990 Current protocols in molecular biology (New York: John Wiley & Sons)
Bagnarol E, Popovici J, Alloisio N, Marechal J, Pujic P, Normand P and Fernandez MP 2007 Differential Frankia protein patterns induced by phenolic extracts from Myricaceae seeds. Physiol. Plant. 130 380–390
Becker A, Schmidt M, Jager W and Puhler A 1995 New gentamicin-resistance and lacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Gene 162 37–39
Benson DR and Silvester WB 1993 Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 57 293–319
Cournoyer B and Normand P 1992 Electropermeabilization of Frankia intact cells to plasmid DNA. Acta Œcologica 13 369–378
Fuhrmann M, Oertel W and Hegemann P 1999 A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J. 19 353–361
Huss-Danell K 1997 Actinorhizal symbioses and their N2 fixation. New Phytol. 136 375–405
Ikemura T 1981 Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J. Mol. Biol. 151 389–409
Kucho K, Hay AE and Normand P 2010 The determinants of the actinorhizal symbiosis. Microbes Environ. 25 241–252
Kucho K, Kakoi K, Yamaura M, Higashi S, Uchiumi T and Abe M 2009 Transient transformation of Frankia by fusion marker genes in liquid culture. Microbes Environ. 24 231–240
Kudla G, Murray AW, Tollervey D and Plotkin JB 2009 Coding-sequence determinants of gene expression in Escherichia coli. Science 324 255–258
Mastronunzio JE, Huang Y and Benson DR 2009 Diminished exoproteome of Frankia spp. in culture and symbiosis. Appl. Environ. Microbiol. 75 6721–6728
Mastronunzio JE, Tisa LS, Normand P and Benson DR 2008 Comparative secretome analysis suggests low plant cell wall degrading capacity in Frankia symbionts. BMC Genomics 9 47
Matsuo T, Onai K, Okamoto K, Minagawa J and Ishiura M 2006 Real-time monitoring of chloroplast gene expression by a luciferase reporter: evidence for nuclear regulation of chloroplast circadian period. Mol. Cell Biol. 26 863–870
Mullin BC and An CS 1990 The molecular genetics of Frankia; in The biology of Frankia and Actinorhizal plants (eds) CR Schwintzer and JD Tjepkema (San Diego: Academic Press) pp 195–214
Myers AK and Tisa LS 2003 Effect of electroporation conditions on cell viability of Frankia EuI1c. Plant Soil 254 83–88
Normand P, Lapierre P, Tisa LS, Gogarten JP, Alloisio N, Bagnarol E, Bassi CA, Berry AM, et al. 2007 Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res. 17 7–15
Sen A, Sur S, Bothra AK, Benson DR, Normand P and Tisa LS 2008 The implication of life style on codon usage patterns and predicted highly expressed genes for three Frankia genomes. Anton. van Leeuw. 93 335–346
Sharp PM and Li WH 1987 The codon adaptation index - a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15 1281–1295
Simonet P, Normand P, Hirsch AM and Akkermans ADL 1990 The genetics of the Frankia-actinorhizal symbiosis; in Molecular biology of symbiotic nitrogen fixation (ed) PM Gresshoff (Boca Raton: CRC Press) pp 77–109
Vervoort EB, van Ravestein A, van Peij NN, Heikoop JC, van Haastert PJ, Verheijden GF and Linskens MH 2000 Optimizing heterologous expression in Dictyostelium: importance of 5' codon adaptation. Nucleic Acids Res. 28 2069–2074
Zhang Z, Lopez MF and Torrey JG 1984 A comparison of cultural characteristics and infectivity of Frankia isolates from root nodules of Casuarina species. Plant Soil 78 79–90
Acknowledgements
We thank Dr Louis S Tisa (University of New Hampshire) for generously providing the Frankia sp. strain CcI3. This work was supported by grants from Nissan Science Foundation, The Asahi Glass Foundation and Kagoshima Kagaku Kenkyuusyo, and MEXT KAKENHI 21770053.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Kucho K, Kakoi K, Yamaura M, Iwashita M, Abe M and Uchiumi T 2013 Codon-optimized antibiotic resistance gene improves efficiency of transient transformation in Frankia. J. Biosci. 38 1–5] DOI 10.1007/s12038-013-9361-4
Rights and permissions
About this article
Cite this article
Kucho, Ki., Kakoi, K., Yamaura, M. et al. Codon-optimized antibiotic resistance gene improves efficiency of transient transformation in Frankia . J Biosci 38, 713–717 (2013). https://doi.org/10.1007/s12038-013-9361-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-013-9361-4