Abstract
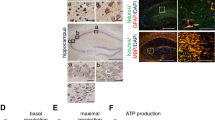
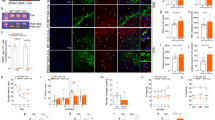
Nicotinamide phosphoribosyltransferase (NAMPT) is the key enzyme in the salvaging synthesis pathway of the nicotinamide adenine dinucleotide (NAD). Both NAMPT and NAD progressively decline upon aging and neurodegenerative diseases. The depletion of NAMPT induces mitochondrial dysfunction in motor neurons and causes bioenergetic stress in neurons. However, the roles of NAMPT in hippocampus neurons need to be further studied. Using floxed Nampt (Namptflox/flox) mice, we knocked out Nampt specifically in the hippocampus CA1 neurons by injecting rAAV-hSyn-Cre-APRE-pA. The depletion of NAMPT in hippocampus neurons induced cognitive deficiency in mice. Nevertheless, no morphological change of hippocampus neurons was observed with immunofluorescent imaging. Under the transmission electron microscope, we observed mitochondrial swollen and mitochondrial number decreasing in the cell body and the neurites of hippocampus neurons. In addition, we found the intracellular Aβ (6E10) increased in the hippocampus CA1 region. The intensity of Aβ42 remained unchanged, but it tended to aggregate. The GFAP level, an astrocyte marker, and the Iba1 level, a microglia marker, significantly increased in the mouse hippocampus. In the primary cultured rat neurons, NAMPT inhibition by FK866 decreased the NAD level of neurons at > 10−9 M. FK866 dropped the mitochondrial membrane potential in the cell body of neurons at > 10−9 M and in the dendrite of neurons at > 10−8 M. FK866 decreased the number and shortened the length of branches of neurons at > 10−7 M. Together, likely due to the injury of mitochondria, the decline of NAMPT level can be a critical risk factor for neurodegeneration.






Similar content being viewed by others
Data Availability
The complete datasets in the current study are available from the corresponding author on request.
References
Zeng K, Yu X, Mahaman YAR, Wang JZ, Liu R, Li Y, Wang X (2022) Defective mitophagy and the etiopathogenesis of Alzheimer’s disease. Transl Neurodegener 11:32. https://doi.org/10.1186/s40035-022-00305-1
Yaku K, Okabe K, Nakagawa T (2018) NAD metabolism: implications in aging and longevity. Ageing Res Rev 47:1–17. https://doi.org/10.1016/j.arr.2018.05.006
Okabe K, Yaku K, Tobe K, Nakagawa T (2019) Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci 26:34. https://doi.org/10.1186/s12929-019-0527-8
Clement J, Wong M, Poljak A, Sachdev P, Braidy N (2019) The plasma NAD(+) metabolome is dysregulated in “normal” aging. Rejuvenation Res 22:121–130. https://doi.org/10.1089/rej.2018.2077
Ralto KM, Rhee EP, Parikh SM (2020) NAD(+) homeostasis in renal health and disease. Nat Rev Nephrol 16:99–111. https://doi.org/10.1038/s41581-019-0216-6
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F (2002) Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol 32:3225–3234. https://doi.org/10.1002/1521-4141(200211)32:11%3c3225::aid-immu3225%3e3.0.co;2-l
Rajman L, Chwalek K, Sinclair DA (2018) Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab 27:529–547. https://doi.org/10.1016/j.cmet.2018.02.011
Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18:416–430. https://doi.org/10.1016/j.cmet.2013.07.013
Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L et al (2018) De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 563:354–359. https://doi.org/10.1038/s41586-018-0645-6
Xie X, Gao Y, Zeng M, Wang Y, Wei TF, Lu YB, Zhang WP (2019) Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis 34:353–366. https://doi.org/10.1007/s11011-018-0346-8
Liu L, Su X, Quinn WJ 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L et al (2018) Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 27(1067–1080):e5. https://doi.org/10.1016/j.cmet.2018.03.018
Lautrup S, Sinclair DA, Mattson MP, Fang EF (2019) NAD(+) in brain aging and neurodegenerative disorders. Cell Metab 30:630–655. https://doi.org/10.1016/j.cmet.2019.09.001
Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, Imai S (2014) Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J Neurosci 34:5800–5815. https://doi.org/10.1523/JNEUROSCI.4730-13.2014
Johnson S, Wozniak DF, Imai S (2018) CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech Dis 4:10. https://doi.org/10.1038/s41514-018-0029-z
Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A et al (2017) Deletion of Nampt in projection neurons of adult mice leads to motor dysfunction, neurodegeneration, and death. Cell Rep 20:2184–2200. https://doi.org/10.1016/j.celrep.2017.08.022
Lundt S, Zhang N, Li JL, Zhang Z, Zhang L, Wang X, Bao R, Cai F et al (2021) Metabolomic and transcriptional profiling reveals bioenergetic stress and activation of cell death and inflammatory pathways in vivo after neuronal deletion of NAMPT. J Cereb Blood Flow Metab 41:2116–2131. https://doi.org/10.1177/0271678X21992625
Alexandris AS, Ryu J, Rajbhandari L, Harlan R, McKenney J, Wang Y, Aja S, Graham D et al (2022) Protective effects of NAMPT or MAPK inhibitors and NaR on Wallerian degeneration of mammalian axons. Neurobiol Dis 171:105808. https://doi.org/10.1016/j.nbd.2022.105808
Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S (2010) Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab 30:1962–1971. https://doi.org/10.1038/jcbfm.2010.71
Chini CCS, Tarrago MG, Chini EN (2017) NAD and the aging process: role in life, death and everything in between. Mol Cell Endocrinol 455:62–74. https://doi.org/10.1016/j.mce.2016.11.003
Wen Y, Parrish JZ, He R, Zhai RG, Kim MD (2011) Nmnat exerts neuroprotective effects in dendrites and axons. Mol Cell Neurosci 48:1–8. https://doi.org/10.1016/j.mcn.2011.05.002
Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J (2016) NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. Elife 5.https://doi.org/10.7554/eLife.19749
Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH (2016) Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352:1474–1477. https://doi.org/10.1126/science.aad5168
Guberovic I, Hurtado-Bages S, Rivera-Casas C, Knobloch G, Malinverni R, Valero V, Leger MM, Garcia J et al (2021) Evolution of a histone variant involved in compartmental regulation of NAD metabolism. Nat Struct Mol Biol 28:1009–1019. https://doi.org/10.1038/s41594-021-00692-5
Canto C, Menzies KJ, Auwerx J (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22:31–53. https://doi.org/10.1016/j.cmet.2015.05.023
Reddy PH, Oliver DM (2019) Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer's disease. Cells 8.https://doi.org/10.3390/cells8050488
Khatoon R, Pahuja M, Parvez S (2020) Cross talk between mitochondria and other targets in Alzheimer’s disease. J Environ Pathol Toxicol Oncol 39:261–279. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2020034249
Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A, Chan H, Bouchet-Marquis C et al (2022) Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat Neurosci 25:688–701. https://doi.org/10.1038/s41593-022-01084-8
Picca A, Calvani R, Coelho-Junior HJ, Landi F, Bernabei R, Marzetti E (2020) Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants (Basel) 9:647. https://doi.org/10.3390/antiox9080647
Hinarejos I, Machuca-Arellano C, Sancho P, Espinos C (2020) Mitochondrial Dysfunction, Oxidative Stress and Neuroinflammation in Neurodegeneration with Brain Iron Accumulation (NBIA). Antioxidants (Basel) 9:1020. https://doi.org/10.3390/antiox9101020
Acknowledgements
We acknowledge Prof. Tang C for the critical reading of this manuscript.
Funding
The work was supported by the National Key R&D Program of China (2018YFA0507700), the National Natural Science Foundation of China (81971304 and 81573400), and the Zhejiang Provincial Natural Science Foundation of China (LY18H170001, LZ20H010001).
Author information
Authors and Affiliations
Contributions
WPZ and YBL designed the research, analyzed and interpreted data, and wrote the manuscript. CS performed the behavioral tests. CC performed the AAV injection and cultured the neurons. TW and TYG prepared and genotyped the mice. CS, CC, TW, TYG, and MZ prepared the materials, performed the experiments, and collected and preliminary analyzed the data together. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments and animal husbandry were performed according to a protocol approved by the Ethics Committee of Laboratory Animal Care and Welfare, Zhejiang University School of Medicine, with the approval number ZJU2015-012–02.
Consent to Participate
Not applicable.
Consent for Publication
All authors approved the publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, C., Chen, C., Wang, T. et al. The Depletion of NAMPT Disturbs Mitochondrial Homeostasis and Causes Neuronal Degeneration in Mouse Hippocampus. Mol Neurobiol 60, 1267–1280 (2023). https://doi.org/10.1007/s12035-022-03142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03142-5