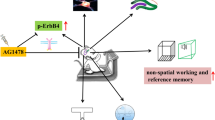
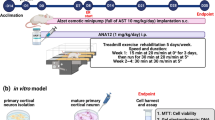
Abstract
Single-factor intervention, such as physical exercise and auditory and visual stimulation, plays a positive role on the prevention and treatment of Alzheimer’s disease (AD); however, the therapeutic effects of single-factor intervention are limited. The beneficial effects of these multifactor combinations on AD and its molecular mechanism have yet to be elucidated. Here, we investigated the effect of multifactor intervention, voluntary wheel exercise, and involuntary treadmill running in combination with acousto-optic stimulation, on adult neurogenesis and behavioral phenotypes in a mouse model of AD. We found that 4 weeks of multifactor intervention can significantly increase the production of newborn cells (BrdU+ cells) and immature neurons (DCX+ cells) in the hippocampus and lateral ventricle of Aβ oligomer-induced mice. Importantly, the multifactor intervention could promote BrdU+ cells to differentiate into neurons (BrdU+ DCX+ cells or BrdU+ NeuN+ cells) and astrocytes (BrdU+GFAP+ cells) in the hippocampus and ameliorate Aβ oligomer-induced cognitive impairment and anxiety- and depression-like behaviors in mice evaluated by novel object recognition, Morris water maze tests, elevated zero maze, forced swimming test, and tail suspension test, respectively. Moreover, multifactor intervention could lead to an increase in the protein levels of PSD-95, SYP, DCX, NeuN, GFAP, Bcl-2, BDNF, TrkB, and pSer473-Akt and a decrease in the protein levels of BAX and caspase-9 in the hippocampal lysates of Aβ oligomer-induced mice. Furthermore, sequencing analysis of serum metabolites revealed that aberrantly expressed metabolites modulated by multifactor intervention were highly enriched in the biological process associated with keeping neurons functioning and neurobehavioral function. Additionally, the intervention-mediated serum metabolites mainly participated in glutamate metabolism, glucose metabolism, and the tricarboxylic acid cycle in mice. Our findings suggest the potential of multifactor intervention as a non-invasive therapeutic strategy for AD to anti-Aβ oligomer neurotoxicity.
Graphical abstract












Similar content being viewed by others
Data Availability
The data generated during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid-beta
- NBM:
-
Nucleus basalis magnocellularis
- PSD-95:
-
Postsynaptic density 95
- SYP:
-
Synaptophysin
- DCX:
-
Doublecortin
- NeuN:
-
Neuronal nuclei
- GFAP:
-
Glial fibrillary acidic protein
- Bcl-2:
-
B cell chronic lymphoma-2
- ICR:
-
Institute of Cancer Research
- NIH:
-
National Institutes of Health
- HFIP:
-
Hexafluoroisopropanol
- LED:
-
Light-emitting diode
- i.p.:
-
Intraperitoneal
- BrdU:
-
5′-Bromo-2′-deoxyuridine
- aNSCs:
-
Adult neural stem cells
- PBS:
-
Phosphate-buffered saline
- PFA:
-
Paraformaldehyde
- DAPI:
-
4′6-Diamidino-2-phenylindole
- MWM:
-
Morris water maze
- NOR:
-
Novel object recognition
- EZM:
-
Elevated zero maze
- FST:
-
Forced swimming test
- TST:
-
Tail suspension test
- PVDF:
-
Polyvinylidene difluoride membranes
- GC-MS:
-
Gas chromatography-mass spectrometry
- EDTA:
-
Ethylenediaminetetraacetic acid
- QC:
-
Quality control
- CAMERA:
-
Collection of algorithms of metabolite profile annotation
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least square discriminant analysis
- VIP:
-
Variable importance in the projection
- ANOVA:
-
Analysis of variance
- SGZ:
-
Subgranular zones
- SVZ:
-
Subventricular zone
- DG:
-
Dentate gyrus
- BDNF:
-
Brain-derived neurotrophic factor
- TrkB:
-
Tropomyosin-related kinase receptor type B
- UHPLC-Q-TOF/MS:
-
Ultra-performance liquid chromatography and quadrupole time-of-flight/metabolomics
- OPLS-DA:
-
Orthogonal partial least-squares discriminant analysis
References
Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, von Arnim CAF, Tousseyn T, Balusu S, De Strooper B, Thal DR (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 139(3):463–484. https://doi.org/10.1007/s00401-019-02103-y
Imamura T, Yanagihara YT, Ohyagi Y, Nakamura N, Iinuma KM, Yamasaki R, Asai H, Maeda M, Murakami K, Irie K, Kira JI (2020) Insulin deficiency promotes formation of toxic amyloid-beta42 conformer co-aggregating with hyper-phosphorylated tau oligomer in an Alzheimer’s disease model. Neurobiol Dis 137:104739. https://doi.org/10.1016/j.nbd.2020.104739
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120(4):545–555. https://doi.org/10.1016/j.cell.2005.02.008
Mahgoub N, Alexopoulos GS (2016) Amyloid hypothesis: is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry 24(3):239–247. https://doi.org/10.1016/j.jagp.2015.12.003
International AsD (2019) World Alzheimer's report 2019: attitudes to dementia. London: Alzheimer's Disease International. https://www.alzint.org/resource/world-alzheimer-report-2019. Accessed 20 Sept 2019
Alzheimer’s A (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12(4):459–509. https://doi.org/10.1016/j.jalz.2016.03.001
Bossers WJ, van der Woude LH, Boersma F, Hortobagyi T, Scherder EJ, van Heuvelen MJ (2016) Comparison of effect of two exercise programs on activities of daily living in individuals with dementia: a 9-week randomized, controlled trial. J Am Geriatr Soc 64(6):1258–1266. https://doi.org/10.1111/jgs.14160
Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25(4):554–560. https://doi.org/10.1038/s41591-019-0375-9
Weller J, Budson A (2018) Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res(7):F1000. https://doi.org/10.12688/f1000research.14506.1
Kempermann G (2019) Environmental enrichment, new neurons and the neurobiology of individuality. Nat Rev Neurosci 20(4):235–245. https://doi.org/10.1038/s41583-019-0120-x
Triviño-Paredes J, Patten AR, Gil-Mohapel J, Christie BR (2016) The effects of hormones and physical exercise on hippocampal structural plasticity. Front Neuroendocrinol 41:23–43. https://doi.org/10.1016/j.yfrne.2016.03.001
Wu C, Yang L, Tucker D, Dong Y, Zhu L, Duan R, Liu TC, Zhang Q (2018) Beneficial effects of exercise pretreatment in a sporadic Alzheimer’s rat model. Med Sci Sports Exerc 50(5):945–956. https://doi.org/10.1249/mss.0000000000001519
Cassé-Perrot C, Lanteaume L, Deguil J, Bordet R, Auffret A, Otten L, Blin O, Bartrés-Faz D, Micallef J (2016) Neurobehavioral and cognitive changes induced by sleep deprivation in healthy volunteers. CNS Neurol Disord Drug Targets 15(7):777–801. https://doi.org/10.2174/1871527315666160518125156
Yu F, Vock DM, Barclay TR (2018) Executive function: responses to aerobic exercise in Alzheimer’s disease. Geriatr Nurs 39(2):219–224. https://doi.org/10.1016/j.gerinurse.2017.09.005
Soni M, Orrell M, Bandelow S, Steptoe A, Rafnsson S, d’Orsi E, Xavier A, Hogervorst E (2019) Physical activity pre- and post-dementia: English longitudinal study of ageing. Aging Ment Health 23(1):15–21. https://doi.org/10.1080/13607863.2017.1390731
Hosseini N, Alaei H, Reisi P, Radahmadi M (2013) The effect of treadmill running on memory before and after the NBM-lesion in rats. J Bodyw Mov Ther 17(4):423–429. https://doi.org/10.1016/j.jbmt.2012.12.005
Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW (2009) Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement 5(4):287–294. https://doi.org/10.1016/j.jalz.2009.02.006
Koo JH, Kang EB, Oh YS, Yang DS, Cho JY (2017) Treadmill exercise decreases amyloid-beta burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp Neurol 288:142–152. https://doi.org/10.1016/j.expneurol.2016.11.014
Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q (2017) Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. J Alzheimers Dis 56(4):1469–1484. https://doi.org/10.3233/jad-160869
Voss MW, Vivar C, Kramer AF, van Praag H (2013) Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17(10):525–544. https://doi.org/10.1016/j.tics.2013.08.001
Hötting K, Röder B (2013) Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 37(9 Pt B):2243–2257. https://doi.org/10.1016/j.neubiorev.2013.04.005
de Oliveira SF, Ferreira JV, Plácido J, Sant’Anna P, Araújo J, Marinho V, Laks J, Camaz Deslandes A (2019) Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: a randomized controlled trial. Maturitas 126:28–33. https://doi.org/10.1016/j.maturitas.2019.04.217
Karssemeijer EGA, Aaronson JA, Bossers WJR, Donders R, Olde Rikkert MGM, Kessels RPC (2019) The quest for synergy between physical exercise and cognitive stimulation via exergaming in people with dementia: a randomized controlled trial. Alzheimers Res Ther 11(1):3. https://doi.org/10.1186/s13195-018-0454-z
Schimidt HL, Garcia A, Izquierdo I, Mello-Carpes PB, Carpes FP (2019) Strength training and running elicit different neuroprotective outcomes in a β-amyloid peptide-mediated Alzheimer’s disease model. Physiol Behav 206:206–212. https://doi.org/10.1016/j.physbeh.2019.04.012
Kurudenkandy FR, Zilberter M, Biverstal H, Presto J, Honcharenko D, Stromberg R, Johansson J, Winblad B, Fisahn A (2014) Amyloid-beta-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. J Neurosci 34(34):11416–11425. https://doi.org/10.1523/JNEUROSCI.1195-14.2014
Zheng C, Bieri KW, Hsiao YT, Colgin LL (2016) Spatial sequence coding differs during slow and fast gamma rhythms in the hippocampus. Neuron 89(2):398–408. https://doi.org/10.1016/j.neuron.2015.12.005
Mably AJ, Gereke BJ, Jones DT, Colgin LL (2017) Impairments in spatial representations and rhythmic coordination of place cells in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 27(4):378–392. https://doi.org/10.1002/hipo.22697
Jafari Z, Kolb BE, Mohajerani MH (2020) Neural oscillations and brain stimulation in Alzheimer’s disease. Prog Neurobiol 194:101878. https://doi.org/10.1016/j.pneurobio.2020.101878
Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S (2019) Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun 10(1):5322. https://doi.org/10.1038/s41467-019-13260-9
Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim DN, Kritskiy O, Barker SJ, Mangena V, Prince SM, Brown EN, Chung K, Boyden ES, Singer AC, Tsai LH (2019) Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177(2):256-271.e222. https://doi.org/10.1016/j.cell.2019.02.014
Park SS, Park HS, Kim CJ, Kang HS, Kim DH, Baek SS, Kim TW (2020) Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimers Res Ther 12(1):62. https://doi.org/10.1186/s13195-020-00631-4
Sun MK (2018) Roles of neural regeneration in memory pharmacology. Neural Regen Res 13(3):406–407. https://doi.org/10.4103/1673-5374.228714
Kang E, Wen Z, Song H, Christian KM, Ming GL (2016) Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol 8(9):a019026. https://doi.org/10.1101/cshperspect.a019026
Weissleder C, North HF, Shannon Weickert C (2019) Important unanswered questions about adult neurogenesis in schizophrenia. Curr Opin Psychiatry 32(3):170–178. https://doi.org/10.1097/yco.0000000000000501
Berger T, Lee H, Young AH, Aarsland D, Thuret S (2020) Adult hippocampal neurogenesis in major depressive disorder and Alzheimer’s disease. Trends Mol Med 26(9):803–818. https://doi.org/10.1016/j.molmed.2020.03.010
Yang H, Luo Y, Hu Q, Tian X, Wen H (2021) Benefits in Alzheimer’s disease of sensory and multisensory stimulation. J Alzheimers Dis 82(2):463–484. https://doi.org/10.3233/JAD-201554
Xiang S, Liu F, Lin J, Chen H, Huang C, Chen L, Zhou Y, Ye L, Zhang K, Jin J, Zhen J, Wang C, He S, Wang Q, Cui W, Zhang J (2017) Fucoxanthin inhibits beta-amyloid assembly and attenuates beta-amyloid oligomer-induced cognitive impairments. J Agric Food Chem 65(20):4092–4102. https://doi.org/10.1021/acs.jafc.7b00805
Siteneski A, Olescowicz G, Pazini FL, Camargo A, Fraga DB, Brocardo PS, Gil-Mohapel J, Cunha MP, Rodrigues ALS (2020) Antidepressant-like and pro-neurogenic effects of physical exercise: the putative role of FNDC5/irisin pathway. J Neural Transm (Vienna) 127(3):355–370. https://doi.org/10.1007/s00702-020-02143-9
Li L, Miao M, Chen J, Liu Z, Li W, Qiu Y, Xu S, Wang Q (2020) Role of ten eleven translocation-2 (Tet2) in modulating neuronal morphology and cognition in a mouse model of Alzheimer’s disease. J Neurochem 157:993–1012. https://doi.org/10.1111/jnc.15234
Li L, Qiu Y, Miao M, Liu Z, Li W, Zhu Y, Wang Q (2020) Reduction of Tet2 exacerbates early stage Alzheimer’s pathology and cognitive impairments in 2×Tg-AD mice. Hum Mol Genet 29(11):1833–1852. https://doi.org/10.1093/hmg/ddz282
Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM (2017) Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci 40(6):347–357. https://doi.org/10.1016/j.tins.2017.04.002
Gusel’nikova VV, Korzhevskiy DE (2015) NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta naturae 7(2):42–47. https://doi.org/10.32607/20758251-2015-7-2-42-47
Duan W, Zhang YP, Hou Z, Huang C, Zhu H, Zhang CQ, Yin Q (2016) Novel insights into NeuN: from neuronal marker to splicing regulator. Mol Neurobiol 53(3):1637–1647. https://doi.org/10.1007/s12035-015-9122-5
Li D, Liu X, Liu T, Liu H, Tong L, Jia S, Wang YF (2020) Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia 68(5):878–897. https://doi.org/10.1002/glia.23734
Ebrahim AS, Sabbagh H, Liddane A, Raufi A, Kandouz M, Al-Katib A (2016) Hematologic malignancies: newer strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol 142(9):2013–2022. https://doi.org/10.1007/s00432-016-2144-1
An HK, Chung KM, Park H, Hong J, Gim JE, Choi H, Lee YW, Choi J, Mun JY, Yu SW (2020) CASP9 (caspase 9) is essential for autophagosome maturation through regulation of mitochondrial homeostasis. Autophagy 16(9):1598–1617. https://doi.org/10.1080/15548627.2019.1695398
Song M, Martinowich K, Lee FS (2017) BDNF at the synapse: why location matters. Mol Psychiatry 22(10):1370–1375. https://doi.org/10.1038/mp.2017.144
Jin W (2020) Regulation of BDNF-TrkB signaling and potential therapeutic strategies for Parkinson’s disease. J Clin Med 9(1):257. https://doi.org/10.3390/jcm9010257
Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, Neal JW, Holmes C, Boche D (2019) Persistent neuropathological effects 14 years following amyloid-beta immunization in Alzheimer’s disease. Brain 142(7):2113–2126. https://doi.org/10.1093/brain/awz142
Brautigam H, Steele JW, Westaway D, Fraser PE, St George-Hyslop PH, Gandy S, Hof PR, Dickstein DL (2012) The isotropic fractionator provides evidence for differential loss of hippocampal neurons in two mouse models of Alzheimer’s disease. Mol Neurodegener 7:58. https://doi.org/10.1186/1750-1326-7-58
Lee SJ, Nam E, Lee HJ, Savelieff MG, Lim MH (2017) Towards an understanding of amyloid-β oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev 46(2):310–323. https://doi.org/10.1039/c6cs00731g
Viola KL, Klein WL (2015) Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 129(2):183–206. https://doi.org/10.1007/s00401-015-1386-3
Glabe CG (2008) Structural classification of toxic amyloid oligomers. J Biol Chem 283(44):29639–29643. https://doi.org/10.1074/jbc.R800016200
Jarosz-Griffiths HH, Noble E, Rushworth JV, Hooper NM (2016) Amyloid-β receptors: the good, the bad, and the prion protein. J Biol Chem 291(7):3174–3183. https://doi.org/10.1074/jbc.R115.702704
Forny-Germano L, Lyra e Silva NM, Batista AF, Brito-Moreira J, Gralle M, Boehnke SE, Coe BC, Lablans A, Marques SA, Martinez AM, Klein WL, Houzel JC, Ferreira ST, Munoz DP, De Felice FG, (2014) Alzheimer’s disease-like pathology induced by amyloid-β oligomers in nonhuman primates. J Neurosci 34(41):13629–13643. https://doi.org/10.1523/jneurosci.1353-14.2014
Morroni F, Sita G, Tarozzi A, Rimondini R, Hrelia P (2016) Early effects of Aβ1-42 oligomers injection in mice: involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav Brain Res 314:106–115. https://doi.org/10.1016/j.bbr.2016.08.002
Prado Lima MG, Schimidt HL, Garcia A, Daré LR, Carpes FP, Izquierdo I, Mello-Carpes PB (2018) Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc Natl Acad Sci U S A 115(10):E2403-e2409. https://doi.org/10.1073/pnas.1718435115
Rossi Dare L, Garcia A, Alves N, Ventura Dias D, de Souza MA, Mello-Carpes PB (2019) Physical and cognitive training are able to prevent recognition memory deficits related to amyloid beta neurotoxicity. Behav Brain Res 365:190–197. https://doi.org/10.1016/j.bbr.2019.03.007
Peterman JL, White JD, Calcagno A, Hagen C, Quiring M, Paulhus K, Gurney T, Eimerbrink MJ, Curtis M, Boehm GW, Chumley MJ (2020) Prolonged isolation stress accelerates the onset of Alzheimer’s disease-related pathology in 5xFAD mice despite running wheels and environmental enrichment. Behav Brain Res 379:112366. https://doi.org/10.1016/j.bbr.2019.112366
Koo JH, Kang EB, Oh YS, Yang DS, Cho JY (2017) Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp Neurol 288:142–152. https://doi.org/10.1016/j.expneurol.2016.11.014
De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas AG, Viña J, Gomez-Cabrera MC (2020) Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 9(5):394–404. https://doi.org/10.1016/j.jshs.2020.01.004
Lin Y, Lu X, Dong J, He X, Yan T, Liang H, Sui M, Zheng X, Liu H, Zhao J, Lu X (2015) Involuntary, forced and voluntary exercises equally attenuate neurocognitive deficits in vascular dementia by the BDNF-pCREB mediated pathway. Neurochem Res 40(9):1839–1848. https://doi.org/10.1007/s11064-015-1673-3
Belviranlı M, Okudan N (2019) Voluntary, involuntary and forced exercises almost equally reverse behavioral impairment by regulating hippocampal neurotrophic factors and oxidative stress in experimental Alzheimer’s disease model. Behav Brain Res 364:245–255. https://doi.org/10.1016/j.bbr.2019.02.030
Robison LS, Popescu DL, Anderson ME, Francis N, Hatfield J, Sullivan JK, Beigelman SI, Xu F, Anderson BJ, Van Nostrand WE, Robinson JK (2019) Long-term voluntary wheel running does not alter vascular amyloid burden but reduces neuroinflammation in the Tg-SwDI mouse model of cerebral amyloid angiopathy. J Neuroinflammation 16(1):144. https://doi.org/10.1186/s12974-019-1534-0
Bernardo TC, Beleza J, Rizo-Roca D, Santos-Alves E, Leal C, Martins MJ, Ascensão A, Magalhães J (2020) Physical exercise mitigates behavioral impairments in a rat model of sporadic Alzheimer’s disease. Behav Brain Res 379:112358. https://doi.org/10.1016/j.bbr.2019.112358
Chen WQ, Viidik A, Skalicky M, Höger H, Lubec G (2007) Hippocampal signaling cascades are modulated in voluntary and treadmill exercise rats. Electrophoresis 28(23):4392–4400. https://doi.org/10.1002/elps.200700336
Buzsáki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
Garza KM, Zhang L, Borron B, Wood LB, Singer AC (2020) Gamma Visual stimulation induces a neuroimmune signaling profile distinct from acute neuroinflammation. J Neurosci 40(6):1211–1225. https://doi.org/10.1523/jneurosci.1511-19.2019
He Q, Colon-Motas KM, Pybus AF, Piendel L, Seppa JK, Walker ML, Manzanares CM, Qiu D, Miocinovic S, Wood LB, Levey AI, Lah JJ, Singer AC (2021) A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement (N Y) 7(1):e12178. https://doi.org/10.1002/trc2.12178
Tu S, Okamoto S, Lipton SA, Xu H (2014) Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener 9:48. https://doi.org/10.1186/1750-1326-9-48
Scopa C, Marrocco F, Latina V, Ruggeri F, Corvaglia V, La Regina F, Ammassari-Teule M, Middei S, Amadoro G, Meli G, Scardigli R, Cattaneo A (2020) Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ 27(3):934–948. https://doi.org/10.1038/s41418-019-0409-3
Cameron HA, Glover LR (2015) Adult neurogenesis: beyond learning and memory. Annu Rev Psychol 66:53–81. https://doi.org/10.1146/annurev-psych-010814-015006
Zhao X, van Praag H (2020) Steps towards standardized quantification of adult neurogenesis. Nat Commun 11(1):4275. https://doi.org/10.1038/s41467-020-18046-y
Hüttenrauch M, Brauß A, Kurdakova A, Borgers H, Klinker F, Liebetanz D, Salinas-Riester G, Wiltfang J, Klafki HW, Wirths O (2016) Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Transl Psychiatry 6(5):e800. https://doi.org/10.1038/tp.2016.65
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472(7344):466–470. https://doi.org/10.1038/nature09817
Cope EC, Gould E (2019) Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell 24(5):690–705. https://doi.org/10.1016/j.stem.2019.03.023
Johansson M, Stomrud E, Lindberg O, Westman E, Johansson PM, van Westen D, Mattsson N, Hansson O (2020) Apathy and anxiety are early markers of Alzheimer's disease. Neurobiol Aging https://doi.org/10.1016/j.neurobiolaging.2019.10.008
Chiu PY, Steffens D, Chen PK, Hsu YC, Huang HT, Lai TJ (2012) Depression in Taiwanese patients with Alzheimer’s disease determined by the National Institutes of Mental Health Provisional Criteria. Int Psychogeriatr 24(8):1299–1305. https://doi.org/10.1017/S1041610211002262
Honer WG (2003) Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiol Aging 24(8):1047–1062. https://doi.org/10.1016/j.neurobiolaging.2003.04.005
Zhang C, Zhang Y, Shen Y, Zhao G, Xie Z, Dong Y (2017) Anesthesia/surgery induces cognitive impairment in female Alzheimer’s disease Transgenic Mice. J Alzheimers Dis 57(2):505–518. https://doi.org/10.3233/jad-161268
Wang B, Wu Q, Lei L, Sun H, Michael N, Zhang X, Wang Y, Zhang Y, Ge B, Wu X, Wang Y, Xin Y, Zhao J, Li S (2019) Long-term social isolation inhibits autophagy activation, induces postsynaptic dysfunctions and impairs spatial memory. Exp Neurol 311:213–224. https://doi.org/10.1016/j.expneurol.2018.09.009
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q (2017) Caspase-9: structure, mechanisms and clinical application. Oncotarget 8(14):23996–24008. https://doi.org/10.18632/oncotarget.15098
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23. https://doi.org/10.1038/nrn3379
Nakai T, Nagai T, Tanaka M, Itoh N, Asai N, Enomoto A, Asai M, Yamada S, Saifullah AB, Sokabe M, Takahashi M, Yamada K (2014) Girdin phosphorylation is crucial for synaptic plasticity and memory: a potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J Neurosci 34(45):14995–15008. https://doi.org/10.1523/JNEUROSCI.2228-14.2014
Johnson CH, Ivanisevic J, Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17(7):451–459. https://doi.org/10.1038/nrm.2016.25
Wilkins JM, Trushina E (2017) Application of metabolomics in Alzheimer’s disease. Front Neurol 8:719. https://doi.org/10.3389/fneur.2017.00719
Enache TA, Oliveira-Brett AM (2017) Alzheimer’s disease amyloid beta peptides in vitro electrochemical oxidation. Bioelectrochemistry 114:13–23. https://doi.org/10.1016/j.bioelechem.2016.11.003
Dinamarca MC, Rios JA, Inestrosa NC (2012) Postsynaptic receptors for amyloid-beta oligomers as mediators of neuronal damage in Alzheimer’s disease. Front Physiol 3:464. https://doi.org/10.3389/fphys.2012.00464
Ramires PR, Forjaz CL, Silva ME, Diament J, Nicolau W, Liberman B, Negrão CE (1993) Exercise tolerance is lower in type I diabetics compared with normal young men. Metabolism 42(2):191–195. https://doi.org/10.1016/0026-0495(93)90034-l
Tang X, Liu J, Dong W, Li P, Li L, Lin C, Zheng Y, Hou J, Li D (2013) The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid Based Complement Alternat Med 2013:820695. https://doi.org/10.1155/2013/820695
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583-019-0132-6
Domingues R, Pereira C, Cruz MT, Silva A (2021) Therapies for Alzheimer’s disease: a metabolic perspective. Mol Genet Metab 132(3):162–172. https://doi.org/10.1016/j.ymgme.2021.01.011
Bergau N, Maul S, Rujescu D, Simm A, Navarrete Santos A (2019) Reduction of glycolysis intermediate concentrations in the cerebrospinal fluid of Alzheimer’s disease patients. Front Neurosci 13:871. https://doi.org/10.3389/fnins.2019.00871
Weise CM, Chen K, Chen Y, Kuang X, Savage CR, Reiman EM (2018) Left lateralized cerebral glucose metabolism declines in amyloid-β positive persons with mild cognitive impairment. Neuroimage Clin 20:286–296. https://doi.org/10.1016/j.nicl.2018.07.016
Croteau E, Castellano CA, Fortier M, Bocti C, Fulop T, Paquet N, Cunnane SC (2018) A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol 107:18–26. https://doi.org/10.1016/j.exger.2017.07.004
Acknowledgements
The authors thank the technical support by the Core Facilities, Ningbo University School of Medicine, and the Laboratory Animal Center, Ningbo University.
Funding
This work was supported by National Natural Science Foundation of China (No. 82001155 and No. 32171035), the Natural Science Foundation of Zhejiang Province (No.LQ19H090005 and No.LQ19H090001), Ningbo Science and Technology Bureau (No.2019B10034), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2022KY1144 and No.2019RC316), Scientific Research Fund Project of Ningbo University (XYL20030), the Student Research, Innovation Program of Ningbo University (2021SRIP), and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Contributions
Li-ping Li designed the experiments. Wan-yi Li performed the behavioral experiments. Jun-yan Gao and Su-Yang Lin performed the immunohistochemical experiments. Shao-tao Pan, Biao Xiao, Yu-tao Ma, and Zhi-tao Liu performed the biochemical experiments. Kai Xie, Wei Shen, Qin-wen Wang, Guang-yu Li, and Jie-jie Guo carried out the data analysis. Li-ping Li, Wan-yi Li, and Jun-yan Gao wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978) and approved by the Institutional Animal Care and Use Committee of the Ningbo University School of Medicine (SYXK(Zhe) 2013–0191).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Stimulation alleviates neurogenesis decline caused by Aβ oligomers in mice.
• Stimulation ameliorates Aβ oligomer-induced cognitive deficits in mice.
• Stimulation attenuates Aβ oligomer-induced anxiety- and depression-like behavior in mice.
• Stimulation inhibits Aβ oligomer-induced neurodegeneration through BDNF/TrkB signaling pathway.
• Stimulation improves glutamate metabolism, glycolysis, and tricarboxylic acid cycle in Aβ-induced mice.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Wy., Gao, Jy., Lin, SY. et al. Effects of Involuntary and Voluntary Exercise in Combination with Acousto-Optic Stimulation on Adult Neurogenesis in an Alzheimer's Mouse Model. Mol Neurobiol 59, 3254–3279 (2022). https://doi.org/10.1007/s12035-022-02784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02784-9