Abstract
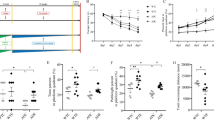
A rat model of vascular dementia was used to compare the effects of involuntary exercise induced by functional electrical stimulation (FES), forced exercise and voluntary exercise on the recovery of cognitive function recovery and its underlying mechanisms. In an involuntary exercise (I-EX) group, FES was used to induce involuntary gait-like running on ladder at 12 m/min. A forced exercise group (F-EX) and a voluntary exercise group (V-EX) exercised by wheel running. The Barnes maze was used for behavioral assessment. Brain-derived neurotrophic factor (BDNF), phosphorylated extracellular signal-regulated kinase 1 and 2 (ERK1/2) and cAMP response element binding protein (CREB) positive cells in hippocampal CA1, CA2/3 and dentate gyrus (DG) regions were evaluated using immunohistochemical methods. Western blotting was used to assess the levels of BDNF, phosphorylated protein kinase B (Akt), tropomyosin receptor kinase B (TrkB), mitogen-activated protein kinase 1 and 2 (MEK1/2), ERK1/2 and CREB in BDNF-pCREB signaling in the hippocampus and prefrontal cortex. Involuntary, forced and voluntary exercises were all found to reverse the cognitive deficits of vascular dementia with about equal effectiveness. The number of BDNF, pCREB and pERK1/2 immunopositive cells was significantly increased in the hippocampal CA1, CA2/3 and DG regions in all three exercise groups. In addition, involuntary exercise activated BDNF and the phosphorylation of Akt, TrkB, MEK1/2, ERK1/2 and CREB in the hippocampus and prefrontal cortex equally as well as voluntary or forced exercise. These results suggest that involuntary exercise induced by FES may be as beneficial for alleviating cognitive deficits after cerebral ischemia.






Similar content being viewed by others
References
Diehl J, Kurz A (2002) Vascular dementias. Fortschr Neurol Psychiatr 70:144–145
Catindig JA, Venketasubramanian N, Ikram MK, Chen C (2012) Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci 321:11–16
Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M (1997) Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rats. Neuroscience 79:1039–1050
Stasiak A, Mussur M, Unzeta M, Lazewska D, Kiec-Kononowicz K, Fogel WA (2011) The central histamine level in rat model of vascular dementia. J Physiol Pharmacol 62:549–558
Cechetti F, Worm PV, Elsner VR, Bertoldi K, Sanches E, Ben J, Siqueira IR, Netto CA (2012) Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem 97:90–96
Arida RM, Scorza FA, Gomes da Silva S, Cysneiros RM, Cavalheiro EA (2011) Exercise paradigms to study brain injury recovery in rodents. Am J Phys Med Rehabil 90:452–465
Cechella JL, Leite MR, Rosario AR, Sampaio TB, Zeni G (2014) Diphenyl diselenide-supplemented diet and swimming exercise enhance novel object recognition memory in old rats. Age (Dordr) 36:9666
Ang ET, Wong PT, Moochhala S, Ng YK (2003) Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience 118:335–345
Shimada H, Hamakawa M, Ishida A, Tamakoshi K, Nakashima H, Ishida K (2013) Low-speed treadmill running exercise improves memory function after transient middle cerebral artery occlusion in rats. Behav Brain Res 243:21–27
Kishi T, Sunagawa K (2012) Exercise training plus calorie restriction causes synergistic protection against cognitive decline via up-regulation of BDNF in hippocampus of stroke-prone hypertensive rats. Conf. Proc IEEE Eng Med Biol Soc 2012:6764–6767
Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D (2005) Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience 136:991–1001
Alomari MA, Khabour OF, Alzoubi KH, Alzubi MA (2013) Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav Brain Res 247:34–39
Xu X, Li Z, Yang Z, Zhang T (2011) Decrease of synaptic plasticity associated with alteration of information flow in a rat model of vascular dementia. Neuroscience 206:136–143
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation.Trends. Neuroscience 30:464–472
Nakajo Y, Miyamoto S, Nakano Y, Xue JH, Hori T, Yanamoto H (2008) Genetic increase in brain-derived neurotrophic factor levels enhances learning and memory. Brain Res 1241:103–109
Murer MG, Yan Q, Raisman-Vozari R (2001) Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol 63:71–124
Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20:709–726
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623
Pérez-Gómez A, Tasker RA (2013) Transient domoic acid excitotoxicity increases BDNF expression and activates both MEK- and PKA-dependent neurogenesis in organotypic hippocampal slices. BMC Neurosci 14:72
Estigoni EH, Fornusek C, Smith RM, Davis GM (2011) Evoked EMG and muscle fatigue during isokinetic FES-cycling in individuals with SCI. Neuromodulation 14:349–355
Hu X, Tong KY, Li R, Chen M, Xue JJ, Ho SK, Chen PN (2010) Combined functional electrical stimulation (FES) and robotic system for wrist rehabilitation after stroke. Stud Health Technol Inform 154:223–228
Foglyano KM, Schnellenberger JR, Kobetic R (2011) Development of a self-contained accelerometry based system for control of functional electrical stimulation in hemiplegia. Conf Proc IEEE Eng Med Biol Soc 2011:5448–5451
Taylor JA, Picard G, Widrick JJ (2011) Aerobic capacity with hybrid FES rowing in spinal cord injury: comparison with arms-only exercise and preliminary findings with regular training. PMR 3:817–824
Ew KH, Wee CL, Zhang DG, Zhu KY, Zheng H (2005) Effects of functional electrical stimulation relating to leg movement. Conf Proc IEEE Eng Med Biol Soc 6:6203–6206
Ring H, Weingarden H (2007) Neuromodulation by functional electrical stimulation (FES) of limb paralysis after stroke. Acta Neurochir Suppl 97:375–380
Yan T, Hui-Chan CW, Li LS (2005) Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke 36:80–85
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Ke Z, Yip SP, Li L, Zheng XX, Tong KY (2011) The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS ONE 6:e16643
Garnier C, Falempin M, Canu MH (2008) A 3D analysis of fore- and hindlimb motion during locomotion: comparison of overground and ladder walking in rats. Behav Brain Res 186:57–65
Whishaw IQ, Travis SG, Koppe SW, Sacrey LA, Gholamrezaei G, Gorny B (2010) Hand shaping in the rat: conserved release and collection vs. flexible manipulation in overground walking, ladder rung walking, cylinder exploration, and skilled reaching. Behav Brain Res 206:21–31
Pang TY, Hannan AJ (2013) Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology 64:515–528
Ji JF, Ji SJ, Sun R, Li K, Zhang Y, Zhang LY, Tian Y (2014) Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem Biophys Res Commun 443:646–651
Zhang L, Zhang J, Sun H, Zhu H, Liu H, Yang Y (2013) An enriched environment elevates corticosteroid receptor levels in the hippocampus and restores cognitive function in a rat model of chronic cerebral hypoperfusion. Pharmacol Biochem Behav 103:693–700
Howlett O, Lannin NA, Ada L, McKinstry C (2015) Functional electrical stimulation improves activity after stroke: a systematic review with meta- analysis. Arch Phys Med Rehabil. doi:10.1016/S0003-9993(15)00044-1
Xiang Y, Liu H, Yan T, Zhuang Z, Jin D, Peng Y (2014) Functional electrical stimulation-facilitated proliferation and regeneration of neural precursor cells in the brains of rats with cerebral infarction. Neural Regen Res 9:243–251
Papathanassoglou ED, Miltiadous P, Karanikola MN (2014) May BDNF be implicated in the exercise-mediated regulation of inflammation? Critical review and synthesis of evidence. Biol Res Nurs. doi:10.1177/1099800414555411
Lu Y, Christian K, Lu B (2007) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323
Aguiar AS Jr, Speck AE, Prediger RD, Kapczinski F, Pinho RA (2008) Downhill training upregulates mice hippocampal and striatal brain-derived neurotrophic factor levels. J Neural Transm 115:1251–1255
Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW (2005) Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133:853–861
Vaynman S, Ying Z, Gomez-Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20:2580–2590
Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G (2011) Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci 33:383–390
Mattson MP (2012) Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 16:706–722
Molteni R, Ying Z, Gómez-Pinilla F (2002) Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci 16:1107–1116
Shen H, Tong L, Balazs R, Cotman CW (2001) Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience 107:219–229
Treves A, Tashiro A, Witter ME, Moser EI (2008) What is the mammalian dentate gyrus good for? Neuroscience 154:1155–1172
McGaugh JL (1966) Time-dependent processes in memory storage. Science 153:1351–1358
Warden MR, Miller EK (2010) Task-dependent changes in short-term memory in the prefrontal cortex. J Neurosci 30:15801–15810
Langdon KD, Granter-Button S, Harley CW, Moody-Corbett F, Peeling J, Corbett D (2014) A cognitive rehabilitation paradigm effective in male rats lacks efficacy in female rats. J Cereb Blood Flow Metab 34:1673–1680
Chen C, Leys D, Esquenazi A (2013) The interaction between neuropsychological and motor deficits in patients after stroke. Neurology 80:S27–S34
Acknowledgments
This work was supported by China’s National Natural Science Foundation (No. 81171863) and the Doctoral Fund of China’s Ministry of Education (No. 20120171110079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Yangyang Lin, Xiao Lu and Juntao Dong have contributed equally to preparing this report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2015_1673_MOESM1_ESM.tif
Supplementary Fig. 1 The three different exercise patterns. (A) A rat in the involuntary exercise group. One end of each electrode has been implanted in the muscle tendon or belly of the digitorum communis muscle (EDC) and the extensor carpi radialis muscle (ECR) on both forelimbs. The other end pierces the skin of the dorsal neck and was connected to the electrical stimulator. (B) A rat in the forced exercise group running at 12 m/min. (C) A rat in the voluntary exercise group running. The arrow indicates a switch counter recording the number of revolutions (TIFF 30901 kb)
Rights and permissions
About this article
Cite this article
Lin, Y., Lu, X., Dong, J. et al. Involuntary, Forced and Voluntary Exercises Equally Attenuate Neurocognitive Deficits in Vascular Dementia by the BDNF–pCREB Mediated Pathway. Neurochem Res 40, 1839–1848 (2015). https://doi.org/10.1007/s11064-015-1673-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1673-3