Abstract
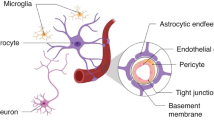
The developing brain is particularly vulnerable to foetal growth restriction (FGR) and abnormal neurodevelopment is common in the FGR infant ranging from behavioural and learning disorders to cerebral palsy. No treatment exists to protect the FGR newborn brain. Recent evidence suggests inflammation may play a key role in the mechanism responsible for the progression of brain impairment in the FGR newborn, including disruption to the neurovascular unit (NVU). We explored whether ibuprofen, an anti-inflammatory drug, could reduce NVU disruption and brain impairment in the FGR newborn. Using a preclinical FGR piglet model, ibuprofen was orally administered for 3 days from birth. FGR brains demonstrated a proinflammatory state, with changes to glial morphology (astrocytes and microglia), and blood-brain barrier disruption, assessed by IgG and albumin leakage into the brain parenchyma and a decrease in blood vessel density. Loss of interaction between astrocytic end-feet and blood vessels was evident where plasma protein leakage was present, suggestive of structural deficits to the NVU. T-cell infiltration was also evident in the parenchyma of FGR piglet brains. Ibuprofen treatment reduced the pro-inflammatory response in FGR piglets, reducing the number of activated microglia and enhancing astrocyte interaction with blood vessels. Ibuprofen also attenuated plasma protein leakage, regained astrocytic end-feet interaction around vessels, and decreased T-cell infiltration into the FGR brain. These findings suggest postnatal administration of ibuprofen modulates the inflammatory state, allowing for stronger interaction between vasculature and astrocytic end-feet to restore NVU integrity. Modulation of the NVU improves the FGR brain microenvironment and may be key to neuroprotection.









Similar content being viewed by others
Availability of Data and Material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Abbreviations
- BBB:
-
Blood-brain barrier
- Cldn:
-
Claudin
- CNS:
-
Central nervous system
- Col IV:
-
Collagen IV
- CXCL10:
-
C-X-C chemokine motif 10
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- CD34:
-
Endothelial progenitor cells
- FGR:
-
Foetal growth restriction
- Gd:
-
Gadolinium
- GFAP:
-
Glial fibrillary astrocytic protein
- Iba-1:
-
Ionised calcium-binding adaptor molecule-1
- Ibu:
-
Ibuprofen
- IgG:
-
Immunoglobulin G
- IL:
-
Interleukin
- MRI:
-
Magnetic resonance imaging
- NVU:
-
Neurovascular unit
- NG:
-
Normally grown
- OCLN:
-
Occludin
- P:
-
Postnatal day
- PBS:
-
Phosphate-buffered saline
- RT:
-
Room temperature
- TJ:
-
Tight junctions
- TNF:
-
Tumour necrosis factor
- ZO-1:
-
Zonula occludens
References
Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL (2019) Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front Endocrinol (Lausanne) 10:55. https://doi.org/10.3389/fendo.2019.00055
Jarvis S, Glinianaia SV, Torrioli MG, Platt MJ, Miceli M, Jouk PS, Johnson A, Hutton J et al (2003) Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet 362(9390):1106–1111. https://doi.org/10.1016/S0140-6736(03)14466-2
Blair EM, Nelson KB (2015) Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks’ gestation. Am J Obstet Gynecol 212(4):520 e521–520 e527. https://doi.org/10.1016/j.ajog.2014.10.1103
Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA (2015) Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics 135(1):126–141. https://doi.org/10.1542/peds.2014-1143
Korzeniewski SJ, Allred EN, Joseph RM, Heeren T, Kuban KCK, O’Shea TM, Leviton A, Investigators ES (2017) Neurodevelopment at age 10 years of children born <28 weeks with fetal growth restriction. Pediatrics 140(5). https://doi.org/10.1542/peds.2017-0697
Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M, Huppi PS (2004) Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56(1):132–138. https://doi.org/10.1203/01.PDR.0000128983.54614.7E
Esteban FJ, Padilla N, Sanz-Cortes M, de Miras JR, Bargallo N, Villoslada P, Gratacos E (2010) Fractal-dimension analysis detects cerebral changes in preterm infants with and without intrauterine growth restriction. NeuroImage 53(4):1225–1232. https://doi.org/10.1016/j.neuroimage.2010.07.019
Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Aden U (2015) Brain growth gains and losses in extremely preterm infants at term. Cerebral cortex 25(7):1897–1905. https://doi.org/10.1093/cercor/bht431
Stolp HB, Dziegielewska KM, Ek CJ, Potter AM, Saunders NR (2005) Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci 22(11):2805–2816. https://doi.org/10.1111/j.1460-9568.2005.04483.x
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV (2018) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21(10):1318–1331. https://doi.org/10.1038/s41593-018-0234-x
Barakat R, Redzic Z (2016) The role of activated microglia and resident macrophages in the neurovascular unit during cerebral ischemia: is the jury still out? Med Princ Pract 25(Suppl 1):3–14. https://doi.org/10.1159/000435858
Mallard C, Ek CJ, Vexler ZS (2018) The myth of the immature barrier systems in the developing brain: role in perinatal brain injury. J Physiol 596(23):5655–5664. https://doi.org/10.1113/JP274938
Wixey JA, Lee KM, Miller SM, Goasdoue K, Colditz PB, Bjorkman ST, Chand KK (2019) Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain. J Neuroinflamm 16(1):5. https://doi.org/10.1186/s12974-018-1392-1
Wixey JA, Sukumar KR, Pretorius R, Lee KM, Colditz PB, Bjorkman ST, Chand KK (2019) Ibuprofen treatment reduces the neuroinflammatory response and associated neuronal and white matter impairment in the growth restricted newborn. Front Physiol 10:541. https://doi.org/10.3389/fphys.2019.00541
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. https://doi.org/10.1038/nrn1824
Jolivel V, Bicker F, Biname F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J et al (2015) Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol 129(2):279–295. https://doi.org/10.1007/s00401-014-1372-1
Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D et al (2019) Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 10(1):5816. https://doi.org/10.1038/s41467-019-13812-z
Castillo-Melendez M, Yawno T, Allison BJ, Jenkin G, Wallace EM, Miller SL (2015) Cerebrovascular adaptations to chronic hypoxia in the growth restricted lamb. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience 45:55–65. https://doi.org/10.1016/j.ijdevneu.2015.01.004
Castillo-Melendez M, Yawno T, Sutherland A, Jenkin G, Wallace EM, Miller SL (2017) Effects of antenatal melatonin treatment on the cerebral vasculature in an ovine model of fetal growth restriction. Dev Neurosci 39(1-4):323–337. https://doi.org/10.1159/000471797
Bell AH, Miller SL, Castillo-Melendez M, Malhotra A (2019) The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci 13:1452. https://doi.org/10.3389/fnins.2019.01452
Ohlsson A, Walia R, Shah SS (2015) Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev 2:CD003481. https://doi.org/10.1002/14651858.CD003481.pub6
Bauer R, Walter B, Brust P, Fuchtner F, Zwiener U (2003) Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur J Obstet Gynecol Reprod Biol 110(Suppl 1):S40–S49
Miller SM, Sullivan SM, Ireland Z, Chand KK, Colditz PB, Bjorkman ST (2016) Neonatal seizures are associated with redistribution and loss of GABAA alpha-subunits in the hypoxic-ischaemic pig. J Neurochem 139(3):471–484. https://doi.org/10.1111/jnc.13746
Bjorkman ST, Miller SM, Rose SE, Burke C, Colditz PB (2010) Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience 166(1):157–167. https://doi.org/10.1016/j.neuroscience.2009.11.067
Radlowski EC, Conrad MS, Lezmi S, Dilger RN, Sutton B, Larsen R, Johnson RW (2014) A neonatal piglet model for investigating brain and cognitive development in small for gestational age human infants. PLoS ONE 9(3):e91951. https://doi.org/10.1371/journal.pone.0091951
Conrad MS, Dilger RN, Johnson RW (2012) Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci 34(4):291–298. https://doi.org/10.1159/000339311
Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3(1):79–83
Kalanjati VP, Wixey JA, Miller SM, Colditz PB, Bjorkman ST (2017) GABAA receptor expression and white matter disruption in intrauterine growth restricted piglets. International Journal of Developmental Neuroscience : the Official Journal of the International Society for Developmental Neuroscience 59:1–9. https://doi.org/10.1016/j.ijdevneu.2017.02.004
Barzilay B, Youngster I, Batash D, Keidar R, Baram S, Goldman M, Berkovitch M, Heyman E (2012) Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 97(2):F116–F119. https://doi.org/10.1136/adc.2011.215160
Kalanjati VP, Miller SM, Ireland Z, Colditz PB, Bjorkman ST (2011) Developmental expression and distribution of GABA(A) receptor alpha1-, alpha3- and beta2-subunits in pig brain. Dev Neurosci 33(2):99–109. https://doi.org/10.1159/000326630
Kuperman VY, Karczmar GS, Blomley MJ, Lewis MZ, Lubich LM, Lipton MJ (1996) Differentiating between T1 and T2* changes caused by gadopentetate dimeglumine in the kidney by using a double-echo dynamic MR imaging sequence. J Magn Reson Imaging 6(5):764–768. https://doi.org/10.1002/jmri.1880060509
Goasdoue K, Chand KK, Miller SM, Lee KM, Colditz PB, Wixey JA, Bjorkman ST (2019) Seizures are associated with blood-brain barrier disruption in a piglet model of neonatal hypoxic-ischaemic encephalopathy. Dev Neurosci:1–16. https://doi.org/10.1159/000499365
Wixey JA, Reinebrant HE, Buller KM (2012) Post-insult ibuprofen treatment attenuates damage to the serotonergic system after hypoxia-ischemia in the immature rat brain. J Neuropathol Exp Neurol 71(12):1137–1148. https://doi.org/10.1097/NEN.0b013e318277d4c7
Bauer R, Walter B, Hoppe A, Gaser E, Lampe V, Kauf E, Zwiener U (1998) Body weight distribution and organ size in newborn swine (sus scrofa domestica) -- a study describing an animal model for asymmetrical intrauterine growth retardation. Exp Toxicol Pathol 50(1):59–65. https://doi.org/10.1016/S0940-2993(98)80071-7
Cox P, Marton T (2009) Pathological assessment of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol 23(6):751–764. https://doi.org/10.1016/j.bpobgyn.2009.06.006
Jain R (2013) Measurements of tumor vascular leakiness using DCE in brain tumors: clinical applications. NMR Biomed 26(8):1042–1049. https://doi.org/10.1002/nbm.2994
LeBleu VS, Macdonald B, Kalluri R (2007) Structure and function of basement membranes. Exp Biol Med (Maywood) 232(9):1121–1129. https://doi.org/10.3181/0703-MR-72
da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR (2014) The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 8:362. https://doi.org/10.3389/fncel.2014.00362
Stanimirovic D, Satoh K (2000) Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain pathology 10(1):113–126. https://doi.org/10.1111/j.1750-3639.2000.tb00248.x
Aly H, Abd-Rabboh L, El-Dib M, Nawwar F, Hassan H, Aaref M, Abdelrahman S, Elsayed A (2009) Ascorbic acid combined with ibuprofen in hypoxic ischemic encephalopathy: a randomized controlled trial. Journal of Perinatology : Official Journal of the California Perinatal Association 29(6):438–443. https://doi.org/10.1038/jp.2009.1
Spindler KR, Hsu TH (2012) Viral disruption of the blood-brain barrier. Trends Microbiol 20(6):282–290. https://doi.org/10.1016/j.tim.2012.03.009
Saunders NR, Liddelow SA, Dziegielewska KM (2012) Barrier mechanisms in the developing brain. Front Pharmacol 3:46. https://doi.org/10.3389/fphar.2012.00046
Carty ML, Wixey JA, Reinebrant HE, Gobe G, Colditz PB, Buller KM (2011) Ibuprofen inhibits neuroinflammation and attenuates white matter damage following hypoxia–ischemia in the immature rodent brain. Brain Res 1402:9–19. https://doi.org/10.1016/j.brainres.2011.06.001
Montagne A, Toga AW, Zlokovic BV (2016) Blood-brain barrier permeability and gadolinium: benefits and potential pitfalls in research. JAMA Neurol 73(1):13–14. https://doi.org/10.1001/jamaneurol.2015.2960
Yurchenco PD, Patton BL (2009) Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15(12):1277–1294. https://doi.org/10.2174/138161209787846766
Urabe N, Naito I, Saito K, Yonezawa T, Sado Y, Yoshioka H, Kusachi S, Tsuji T et al (2002) Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch Histol Cytol 65(2):133–143. https://doi.org/10.1679/aohc.65.133
Webersinke G, Bauer H, Amberger A, Zach O, Bauer HC (1992) Comparison of gene expression of extracellular matrix molecules in brain microvascular endothelial cells and astrocytes. Biochem Biophys Res Commun 189(2):877–884. https://doi.org/10.1016/0006-291x(92)92285-6
Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla HJ (2002) Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res 310(1):19–29. https://doi.org/10.1007/s00441-002-0604-1
Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE (2009) Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114(24):5091–5101. https://doi.org/10.1182/blood-2009-05-222364
Kose N, Asashima T, Muta M, Iizasa H, Sai Y, Terasaki T, Nakashima E (2007) Altered expression of basement membrane-related molecules in rat brain pericyte, endothelial, and astrocyte cell lines after transforming growth factor-beta1 treatment. Drug Metab Pharmacokinet 22(4):255–266. https://doi.org/10.2133/dmpk.22.255
Eldahshan W, Fagan SC, Ergul A (2019) Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res 147:104349. https://doi.org/10.1016/j.phrs.2019.104349
Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T (2018) Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol 38(1):53–71. https://doi.org/10.1007/s10571-017-0504-2
Clark IA, Alleva LM, Vissel B (2010) The roles of TNF in brain dysfunction and disease. Pharmacol Ther 128(3):519–548. https://doi.org/10.1016/j.pharmthera.2010.08.007
Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME (2002) Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 37(3):229–240
Mondo E, Becker SC, Kautzman AG, Schifferer M, Baer CE, Chen J, Huang EJ, Simons M, Schafer DP (2020) A developmental analysis of juxtavascular microglia dynamics and interactions with the vasculature. J Neurosci 40(34):6503–6521. https://doi.org/10.1523/JNEUROSCI.3006-19.2020
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG (2006) Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 37:1087–1093
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58(9):1094–1103. https://doi.org/10.1002/glia.20990
Jing L, He Q, Zhang JZ, Li PA (2013) Temporal profile of astrocytes and changes of oligodendrocyte-based myelin following middle cerebral artery occlusion in diabetic and non-diabetic rats. Int J Biol Sci 9(2):190–199. https://doi.org/10.7150/ijbs.5844
Jing L, Mai L, Zhang JZ, Wang JG, Chang Y, Dong JD, Guo FY, Li PA (2013) Diabetes inhibits cerebral ischemia-induced astrocyte activation - an observation in the cingulate cortex. Int J Biol Sci 9(9):980–988. https://doi.org/10.7150/ijbs.7251
Xu J, Ling EA (1994) Studies of the ultrastructure and permeability of the blood-brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat 184(Pt 2):227–237
Malhotra A, Castillo-Melendez M, Allison BJ, Sutherland AE, Nitsos I, Pham Y, Alves de Alencar Rocha AK, Fahey MC et al (2018) Neuropathology as a consequence of neonatal ventilation in premature growth-restricted lambs. Am J Physiol Regul Integr Comp Physiol 315(6):R1183–R1194. https://doi.org/10.1152/ajpregu.00171.2018
Thurgur H, Pinteaux E (2019) Microglia in the neurovascular unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience 405:55–67. https://doi.org/10.1016/j.neuroscience.2018.06.046
Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y (2010) Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci 112(2):251–254. https://doi.org/10.1254/jphs.09292sc
Yang YM, Shang DS, Zhao WD, Fang WG, Chen YH (2013) Microglial TNF-alpha-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem Res 38(11):2295–2304. https://doi.org/10.1007/s11064-013-1138-5
Kanda H, Kobayashi K, Yamanaka H, Okubo M, Noguchi K (2017) Microglial TNFalpha induces COX2 and PGI2 synthase expression in spinal endothelial cells during neuropathic pain. eNeuro 4(2). https://doi.org/10.1523/ENEURO.0064-17.2017
Krasnow SM, Knoll JG, Verghese SC, Levasseur PR, Marks DL (2017) Amplification and propagation of interleukin-1beta signaling by murine brain endothelial and glial cells. J Neuroinflammation 14(1):133. https://doi.org/10.1186/s12974-017-0908-4
Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, Xia YP, Jin HJ et al (2019) Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 10(7):487. https://doi.org/10.1038/s41419-019-1716-9
Laflamme N, Lacroix S, Rivest S (1999) An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci 19(24):10923–10930
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651
Peng W, Wu X, Feng D, Zhang Y, Chen X, Ma C, Shen H, Li X et al (2020) Cerebral cavernous malformation 3 relieves subarachnoid hemorrhage-induced neuroinflammation in rats through inhibiting NF-kB signaling pathway. Brain Res Bull 160:74–84. https://doi.org/10.1016/j.brainresbull.2020.04.003
Li X, Su L, Zhang X, Zhang C, Wang L, Li Y, Zhang Y, He T et al (2017) Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res 39(4):367–373. https://doi.org/10.1080/01616412.2017.1286541
Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, Hu F, Zhang H (2017) TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol 310:38–45. https://doi.org/10.1016/j.jneuroim.2017.06.006
Muramatsu K, Fukuda A, Togari H, Wada Y, Nishino H (1997) Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood-brain barrier. Stroke 28(11):2281–2288; discussion 2288-2289. https://doi.org/10.1161/01.str.28.11.2281
Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, Shi Y, Leak RK et al (2016) Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol Dis 91:37–46. https://doi.org/10.1016/j.nbd.2016.02.020
Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR (2007) Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci 25(1):231–238. https://doi.org/10.1111/j.1460-9568.2006.05275.x
Michalak Z, Lebrun A, Di Miceli M, Rousset MC, Crespel A, Coubes P, Henshall DC, Lerner-Natoli M, Rigau V (2012) IgG leakage may contribute to neuronal dysfunction in drug-refractory epilepsies with blood-brain barrier disruption. J Neuropathol Exp Neurol 71(9):826–838. https://doi.org/10.1097/NEN.0b013e31826809a6
Malhotra A, Castillo-Melendez M, Allison BJ, Sutherland AE, Nitsos I, Pham Y, McDonald CA, Fahey MC et al (2020) Neurovascular effects of umbilical cord blood-derived stem cells in growth-restricted newborn lambs: UCBCs for perinatal brain injury. Stem Cell Res Ther 11(1):17. https://doi.org/10.1186/s13287-019-1526-0
Laurent C, Dorothee G, Hunot S, Martin E, Monnet Y, Duchamp M, Dong Y, Legeron FP et al (2017) Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 140(1):184–200. https://doi.org/10.1093/brain/aww270
Merlini M, Kirabali T, Kulic L, Nitsch RM, Ferretti MT (2018) Extravascular CD3+ T cells in brains of alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener Dis 18(1):49–56. https://doi.org/10.1159/000486200
Amdi C, Lynegaard JC, Thymann T, Williams AR (2020) Intrauterine growth restriction in piglets alters blood cell counts and impairs cytokine responses in peripheral mononuclear cells 24 days post-partum. Sci Rep 10(1):4683. https://doi.org/10.1038/s41598-020-61623-w
Lewis EL, Tulina N, Anton L, Brown AG, Porrett PM, Elovitz MA (2021) IFNgamma-producing T cells accumulate in the fetal brain following intrauterine inflammation. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.741518
Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonniere L, Larochelle C, Prat A (2015) Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis 74:14–24. https://doi.org/10.1016/j.nbd.2014.09.016
Wu CH, Wang HJ, Wen CY, Lien KC, Ling EA (1997) Response of amoeboid and ramified microglial cells to lipopolysaccharide injections in postnatal rats--a lectin and ultrastructural study. Neurosci Res 27(2):133–141. https://doi.org/10.1016/s0168-0102(96)01140-6
Calvo CF, Yoshimura T, Gelman M, Mallat M (1996) Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci 8(8):1725–1734. https://doi.org/10.1111/j.1460-9568.1996.tb01316.x
Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M (2005) Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr Res 58(4):791–798. https://doi.org/10.1203/01.PDR.0000180535.14093.FB
Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN et al (2017) Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Investig 127(8):3136–3151. https://doi.org/10.1172/JCI91301
Mouser PE, Head E, Ha KH, Rohn TT (2006) Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am J Pathol 168(3):936–946. https://doi.org/10.2353/ajpath.2006.050798
Smith CE, Soti S, Jones TA, Nakagawa A, Xue D, Yin H (2017) Non-steroidal anti-inflammatory drugs are caspase inhibitors. Cell Chem Biol 24(3):281–292. https://doi.org/10.1016/j.chembiol.2017.02.003
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. https://doi.org/10.1083/jcb.200302070
Luissint AC, Federici C, Guillonneau F, Chretien F, Camoin L, Glacial F, Ganeshamoorthy K, Couraud PO (2012) Guanine nucleotide-binding protein Galphai2: a new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J Cereb Blood Flow Metab 32(5):860–873. https://doi.org/10.1038/jcbfm.2011.202
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142. https://doi.org/10.1091/mbc.11.12.4131
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. https://doi.org/10.1124/pr.57.2.4
Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE (2005) Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288(6):C1231–C1241. https://doi.org/10.1152/ajpcell.00581.2004
Sladojevic N, Stamatovic SM, Johnson AM, Choi J, Hu A, Dithmer S, Blasig IE, Keep RF, Andjelkovic AV (2019) Claudin-1-dependent destabilization of the blood-brain barrier in chronic stroke. J Neurosci 39(4):743–757. https://doi.org/10.1523/JNEUROSCI.1432-18.2018
Zhang S, An Q, Wang T, Gao S, Zhou G (2018) Autophagy- and MMP-2/9-mediated reduction and redistribution of ZO-1 contribute to hyperglycemia-increased blood-brain barrier permeability during early reperfusion in stroke. Neuroscience 377:126–137. https://doi.org/10.1016/j.neuroscience.2018.02.035
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596. https://doi.org/10.1038/nm.3407
Acknowledgments
The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Centre for Advanced Imaging, University of Queensland. The authors would also like to thank Lara Jones, Elliot Teo and John Luff for assistance with animal experimentation.
Funding
Financial support was provided by The National Health and Medical Research Council (Australia) (Grant ID: APP1147545), Royal Brisbane and Women’s Hospital Foundation, and Children’s Hospital Foundation (Grant ID 50217) grants.
Author information
Authors and Affiliations
Contributions
KC was involved in obtaining funding and responsible for all laboratory aspects of the project, data analysis, interpretation, writing and editing the manuscript. SM was involved in conducting animal experiments and editing the manuscript. GC was involved in MRI acquisition, data analysis and editing the manuscript. LM undertook all western blotting and edited the manuscript. JP was involved in obtaining funding and editing the manuscript. PC was involved in obtaining funding, critical revision and editing the manuscript. STB was involved in obtaining funding, critical revision and editing the manuscript. JW was involved in obtaining funding, study conception and design, conducting animal experiments, acquiring data, critical revision and writing the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal care, handling and experiments were approved by the University of Queensland Animal Ethics Committee (MED/UQCCR/132/16/RBWH and UQCCR/249/18) and was carried out in accordance with National Health and Medical Research Council (NHMRC) guidelines (Australia) and ARRIVE guidelines.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julie A. Wixey and Stella Tracey Bjorkman should be considered joint senior authors.
Supplementary Information
ESM 1
(DOCX 5861 kb)
Rights and permissions
About this article
Cite this article
Chand, K.K., Miller, S.M., Cowin, G.J. et al. Neurovascular Unit Alterations in the Growth-Restricted Newborn Are Improved Following Ibuprofen Treatment. Mol Neurobiol 59, 1018–1040 (2022). https://doi.org/10.1007/s12035-021-02654-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02654-w