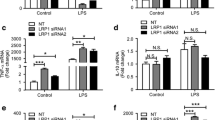
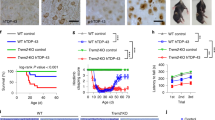
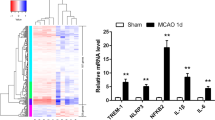
Abstract
Microglial activation-induced neuroinflammation is critical in the pathogenesis of neurodegenerative diseases. Activated microglia are regulated mainly by innate pattern recognition receptors (PRRs) on their surface, of which macrophage receptor with collagenous structure (Marco) is a well-characterized scavenger receptor constitutively expressed on specific subsets of macrophages, including microglia. Increasing evidence has shown that Marco is involved in the pathogenesis of a range of inflammatory processes. However, research on the role of Marco in regulating neuroinflammation has reported conflicting results. In the present study, we examined the role Marco played in triggering neuroinflammation and its underlying mechanisms. The results demonstrated that silencing the Marco gene resulted in a significantly reduced neuroinflammatory response and vice versa. α-Syn stimulation in Marco overexpressing cells induced a pronounced inflammatory response, suggesting that Marco alone could trigger an inflammatory response. We also found that TLR2 significantly promoted Marco-mediated neuroinflammation, indicating TLR2 was an important co-receptor of Marco. Knocking down the TLR2 gene in microglia and mouse substantia nigra resulted in decreased expression of Marco. Subsequent mechanistic studies showed that deleting the SRCR domain of Marco resulted in disruption of the inflammatory response and the interaction between TLR2 and Marco. This suggested that TLR2 binds directly to the SRCR domain of Marco and regulates Marco-mediated neuroinflammation. In summary, this investigation revealed that TLR2 could potentiate Marco-mediated neuroinflammation by interacting with the SRCR domain of Marco, providing a new target for inhibiting neuroinflammation in neurodegenerative diseases.
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PRRs:
-
Pattern recognition receptors
- Marco:
-
Macrophage receptor with collagenous structure
- α-Syn:
-
α-Synuclein
- TLR:
-
Toll-like receptors
- CNS:
-
Central nervous system
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- NLR:
-
NOD-like receptors
- SR:
-
Scavenger receptors
- SRCR:
-
SR cysteine-reach
- NO:
-
Nitric oxide
- Iba-1:
-
Ionized calcium binding adaptor molecule-1
- DAB:
-
Diaminobenzidine
- Co-IP:
-
Co-immunoprecipitation
- MyD88:
-
Myeloid differentiation factor88
- AP-1:
-
Transcription factors activator protein-1
- NF-κB:
-
Nuclear factor kappa-B
- MAPK:
-
Mitogen-activated protein kinase
- LOX-1:
-
Lectin-type oxidized LDL receptor 1
- KpOmpA:
-
Klebsiella pneumoniae
References
Boza-Serrano A, Ruiz R, Sanchez-Varo R et al (2019) Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer's disease. Acta Neuropathol 138(2):251–273
Xu L, He D, Bai Y (2016) Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol 53(10):6709–6715
Sheppard O, Coleman MP, Durrant CS (2019) Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J Neuroinflammation 16(1):106
Gordon S (2002) Pattern recognition receptors: doubling up for the innate immune response. Cell 111(7):927–930
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Mukhopadhyay S, Varin A, Chen Y, Liu B, Tryggvason K, Gordon S (2011) SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood 117(4):1319–1328
Kraal G, van der Laan LJ, Elomaa O, Tryggvason K (2000) The macrophage receptor MARCO. Microbes Infect 2(3):313–316
Sankala M, Brännström A, Schulthess T et al (2002) Characterization of recombinant soluble macrophage scavenger receptor MARCO. J Biol Chem 277(36):33378–33385
Gough PJ, Gordon S (2000) The role of scavenger receptors in the innate immune system. Microbes Infect 2(3):305–311
Elomaa O, Sankala M, Pikkarainen T et al (1998) Structure of the human macrophage MARCO receptor and characterization of its bacteria-binding region. J Biol Chem 273(8):4530–4538
Krieger M, Herz J (1994) Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem 63:601–637
Granucci F, Petralia F, Urbano M et al (2003) The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood 102(8):2940–2947
Józefowski S, Arredouani M, Sulahian T, Kobzik L (2005) Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol 175(12):8032–8041
Benard EL, Roobol SJ, Spaink HP, Meijer AH (2014) Phagocytosis of mycobacteria by zebrafish macrophages is dependent on the scavenger receptor Marco, a key control factor of pro-inflammatory signalling. Dev Comp Immunol 47(2):223–233
Thakur SA, Hamilton RF, Holian A (2008) Role of scavenger receptor a family in lung inflammation from exposure to environmental particles. J Immunotoxicol 5(2):151–157
Plüddemann A, Mukhopadhyay S, Sankala M et al (2009) SR-A, MARCO and TLRs differentially recognise selected surface proteins from Neisseria meningitidis: an example of fine specificity in microbial ligand recognition by innate immune receptors. J Innate Immun 1(2):153–163
Cotena A, Gordon S, Platt N (2004) The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J Immunol 173(10):6427–6432
Haworth R, Platt N, Keshav S et al (1997) The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med 186(9):1431–1439
Acuna L, Corbalán NS, Raisman-Vozari R (2020) Rifampicin quinone pretreatment improves neuronal survival by modulating microglia inflammation induced by α-synuclein. Neural Regen Res 15(8):1473–1474
Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE (2003) A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci 23(7):2665–2674
Arredouani M, Yang Z, Ning Y et al (2004) The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 200(2):267–272
Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE (2007) The nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol 29(3):289–301
Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B (2009) Pivotal advance: Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J Leukoc Biol 85(4):595–605
Kissick HT, Dunn LK, Ghosh S, Nechama M, Kobzik L, Arredouani MS (2014) The scavenger receptor MARCO modulates TLR-induced responses in dendritic cells. PLoS One. 9(8):e104148
Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE (2009) pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem 284(35):23818–23829
Bunk S, Sigel S, Metzdorf D et al (2010) Internalization and coreceptor expression are critical for TLR2-mediated recognition of lipoteichoic acid in human peripheral blood. J Immunol 185(6):3708–3717
Peiser L, De Winther MP, Makepeace K et al (2002) The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect Immun 70(10):5346–5354
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Oliveira-Nascimento L, Massari P, Wetzler LM (2012) The role of TLR2 in infection and immunity. Front Immunol 3:79
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16(1):3–9
Into T, Shibata K (2005) Apoptosis signal-regulating kinase 1-mediated sustained p38 mitogen-activated protein kinase activation regulates mycoplasmal lipoprotein- and staphylococcal peptidoglycan-triggered Toll-like receptor 2 signalling pathways. Cell Microbiol 7(9):1305–1317
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Jeannin P, Bottazzi B, Sironi M et al (2005) Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 22(5):551–560
Hoebe K, Georgel P, Rutschmann S et al (2005) CD36 is a sensor of diacylglycerides. Nature 433(7025):523–527
Arredouani MS, Palecanda A, Koziel H et al (2005) MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol 175(9):6058–6064
Acknowledgements
First, I would like to extend my sincere gratitude to my supervisor, Xiu-Qi Bao and Dan Zhang, for their instructive advice and useful suggestions on my thesis. I am deeply grateful of their help in the completion of this thesis. I am also deeply indebted to all the other tutors and teachers in Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College for their direct and indirect help to me. Special thanks should go to my friends who have put considerable time and effort into their comments on the draft. Finally, I am indebted to my parents for their continuous support and encouragement.
Funding
This work was supported by grants from the National Sciences Foundation of China (81630097, 81773718), CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-3–011), National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the Thirteen Five-year Plan Period (2018ZX09711001-008–005, 2018ZX09711001-003–005, 2018ZX09711001-003–020), and CAMS The Fundamental Research Funds for the Central Universities (2018RC350002).
Author information
Authors and Affiliations
Contributions
LW, X-QB, and DZ contributed to the conception of the study.
LW performed the experiment and data analyses and wrote the manuscript.
H-YY, C-XZ, J-MS, HL, Z-H Z, F-Y Y, CJ, and F-Y L provided help for the experiment.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Efforts were made to minimize animal suffering and to reduce the number of animals used. All animal experiments were performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Yang, HY., Zang, CX. et al. TLR2 Potentiates SR-Marco-Mediated Neuroinflammation by Interacting with the SRCR Domain. Mol Neurobiol 58, 5743–5755 (2021). https://doi.org/10.1007/s12035-021-02463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02463-1