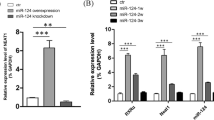
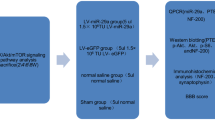
Abstract
LINGO-1(LRR and Ig domain–containing NOGO receptor interacting protein 1) is a viable target for spinal cord injury (SCI) repair due to its potent negative regulation in neuron survival and axonal regeneration. Although promising, the intracellular mechanism underlying LINGO-1 regulation is unclear. Here, we identified miR-615 as a potential microRNA (miRNA) that directly targets LINGO-1 by binding its 3′-untranslated region (3′-UTR) and caused the translation inhibition of LINGO-1. MiR-615 negatively regulated LINGO-1 during neural stem cell (NSC) differentiation and facilitated its neuronal differentiation in vitro. Interestingly, compared to the control, neurons differentiated from miR-615-treated NSCs were immature with short processes. Further results showed LINGO-1/epidermal growth factor receptor (EGFR) signaling may be involved in this process, as blockade of EGFR using specific antagonist resulted in mature neurons with long processes. Furthermore, intrathecal administration of miR-615 agomir in SCI rats effectively knocked down LINGO-1, increased neuronal survival, enhanced axonal extension and myelination, and improved recovery of hindlimbs motor functions. This work thus uncovers miR-615 as an effective miRNA that regulates LINGO-1 in NSC and SCI animals, and suggests miR-615 as a potential therapeutic target for traumatic central nervous system (CNS) injury.






Similar content being viewed by others
References
Yang H, Liu CC, Wang CY, Zhang Q, An J, Zhang L, Hao DJ (2016) Therapeutical strategies for spinal cord injury and a promising autologous astrocyte-based therapy using efficient reprogramming techniques. Mol Neurobiol 53(5):2826–2842. https://doi.org/10.1007/s12035-015-9157-7
Salewski RP, Mitchell RA, Shen C, Fehlings MG (2015) Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev 24(1):36–50
Zhang Z, Xu X, Zhang Y, Zhou J, Yu Z, He C (2009) LINGO-1 interacts with WNK1 to regulate Nogo-induced inhibition of neurite extension. J Biol Chem 284(23):15717–15728. https://doi.org/10.1074/jbc.M808751200
Wu H-F, Cen J-S, Zhong Q, Chen L, Wang J, Deng DY, Wan Y (2013) The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials 34(6):1686–1700
Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF et al (2006) LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci 33(3):311–320. https://doi.org/10.1016/j.mcn.2006.08.003
Mi S, Hu B, Hahm K, Luo Y, Kam HE, Yuan Q, Wong WM, Wang L et al (2007) LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med 13(10):1228–1233. https://doi.org/10.1038/nm1664
Andrews JL, Fernandez-Enright F (2015) A decade from discovery to therapy: Lingo-1, the dark horse in neurological and psychiatric disorders. Neurosci Biobehav Rev 56:97–114. https://doi.org/10.1016/j.neubiorev.2015.06.009
Zhang Y, Zhang YP, Pepinsky B, Huang G, Shields LB, Shields CB, Mi S (2015) Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp Neurol 266:68–73. https://doi.org/10.1016/j.expneurol.2015.02.006
Lööv C, Fernqvist M, Walmsley A, Marklund N, Erlandsson A (2012) Neutralization of LINGO-1 during in vitro differentiation of neural stem cells results in proliferation of immature neurons. PLoS One 7(1):e29771. https://doi.org/10.1371/journal.pone.0029771.g001
Chen N, Cen JS, Wang J, Qin G, Long L, Wang L, Wei F, Xiang Q et al (2016) Targeted inhibition of leucine-rich repeat and immunoglobulin domain-containing protein 1 in transplanted neural stem cells promotes neuronal differentiation and functional recovery in rats subjected to spinal cord injury. Crit Care Med 44(3):e146–e157. https://doi.org/10.1097/CCM.0000000000001351
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19(1):92–105. https://doi.org/10.1101/gr.082701.108
Liu NK, Wang XF, Lu QB, Xu XM (2009) Altered MicroRNA expression following traumatic spinal cord injury. Exp Neurol 2(219):424–429. https://doi.org/10.1016/j.expneurol.2009.06.015
Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC (2011) MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience 186:146–160. https://doi.org/10.1016/j.neuroscience.2011.03.063
Hutchison ER, Okun E, Mattson MP (2009) The therapeutic potential of microRNAs in nervous system damage, degeneration, and repair. NeuroMolecular Med 11(3):153–161. https://doi.org/10.1007/s12017-009-8086-x
Hu JZ, Huang JH, Zeng L, Wang G, Cao M (2013) Anti-apoptotic effect of MicroRNA-21 after contusion spinal cord injury in rats. J Neurotrauma 30:1349–1360. https://doi.org/10.1089/neu.2012.2748
Liu G, Keeler BE, Zhukareva V, Houle JD (2010) Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol 226(1):200–206. https://doi.org/10.1016/j.expneurol.2010.08.032 Copyright (c) 2010 Elsevier Inc. All rights reserved
Ji Y, Sun Q, Zhang J, Hu H (2018) MiR-615 inhibits cell proliferation, migration and invasion by targeting EGFR in human glioblastoma. Biochem Biophys Res Commun 499(3):719–726. https://doi.org/10.1016/j.bbrc.2018.03.217
Yang B, Xie R, Wu SN, Gao CC, Yang XZ, Zhou JF (2018) MicroRNA-615-5p targets insulin-like growth factor 2 and exerts tumor-suppressing functions in human esophageal squamous cell carcinoma. Oncol Rep 39(1):255–263. https://doi.org/10.3892/or.2017.6079
Icli B, Wu W, Ozdemir D, Li H, Cheng HS, Haemmig S, Liu X, Giatsidis G et al (2019) MicroRNA-615-5p regulates angiogenesis and tissue repair by targeting AKT/eNOS (protein kinase B/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscler Thromb Vasc Biol 39(7):1458–1474. https://doi.org/10.1161/atvbaha.119.312726
Tripathi R, Saini HK, Rad R, Abreugoodger C, Van DS (2011) Messenger RNA and microRNA profiling during early mouse EB formation. Gene Expr Patterns 11:334–344. https://doi.org/10.1016/j.gep.2011.03.004
Stallings RL, Foley NH, Bray IM, Das S, Buckley PG (2011) MicroRNA and DNA methylation alterations mediating retinoic acid induced neuroblastoma cell differentiation. Semin Cancer Biol 21:283–290. https://doi.org/10.1016/j.semcancer.2011.07.001
Ji HP, Halder D, Mi RC, Jin CC, Lee YS (2012) Expression profiles of miRNAs during ethanol-induced differentiation of neural stem cells. BioChip J 1(6):73–83. https://doi.org/10.1007/s13206-012-6110-y
Nuneziglesias J, Liu CC, Morgan TE, Finch CE, Zhou XJ (2010) Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer's disease cortex reveals altered miRNA regulation. PLoS One 2(5):e8898
Kojima T, Ueda Y, Sato A, Sameshima H, Ikenoue T (2013) Comprehensive gene expression analysis of cerebral cortices from mature rats after neonatal hypoxic-ischemic brain injury. J Mol Neurosci 49:320–327. https://doi.org/10.1007/s12031-012-9830-5
Roshan R, Ghosh T, Scaria V, Pillai B (2009) MicroRNAs: novel therapeutic targets in neurodegenerative diseases. Drug Discov Today 14(23–24):1123–1129. https://doi.org/10.1016/j.drudis.2009.09.009
Jasmin BJ, Campbell RJ, Michel RN (1995) Nerve-dependent regulation of succinate dehydrogenase in junctional and extrajunctional compartments of rat muscle fibres. J Physiol 484(Pt 1):155–164
Tomer R, Ye L, Hsueh B, Deisseroth K (2014) Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc 9(7):1682–1697. https://doi.org/10.1038/nprot.2014.123
Koprivica V, Cho K-S, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E et al (2005) EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310(5745):106–110
Woltering JM, Durston AJ (2008) MiR-10 represses HoxB1a and HoxB3a in zebrafish. PLoS One 3(1):e1396. https://doi.org/10.1371/journal.pone.0001396
Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK et al (2008) Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134(3):521–533. https://doi.org/10.1016/j.cell.2008.07.020
Aranda P, Agirre X, Ballestar E, Andreu EJ, Roman-Gomez J, Prieto I, Martin-Subero JI, Cigudosa JC et al (2009) Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS One 4(11):e7809. https://doi.org/10.1371/journal.pone.0007809
Wang J, Ye Z, Zheng S, Chen L, Wan Y, Deng Y, Yang R (2016) Lingo-1 shRNA and notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons. Brain Res 1634:34–44. https://doi.org/10.1016/j.brainres.2015.11.029
Li X, Zhang Y, Yan Y, Ciric B, Ma CG, Chin J, Curtis M, Rostami A et al (2017) LINGO-1-fc-transduced neural stem cells are effective therapy for chronic stage experimental autoimmune encephalomyelitis. Mol Neurobiol 54(6):4365–4378. https://doi.org/10.1007/s12035-016-9994-z
Wang JJ, Liu C, Shan K, Liu BH, Li XM, Zhang SJ, Zhou RM, Dong R et al (2018) Circular RNA-ZNF609 regulates retinal neurodegeneration by acting as miR-615 sponge. Theranostics 8(12):3408–3415. https://doi.org/10.7150/thno.25156
Gu H, Yu SP, Gutekunst CA, Gross RE, Wei L (2013) Inhibition of the rho signaling pathway improves neurite outgrowth and neuronal differentiation of mouse neural stem cells. Int J Physiol Pathophysiol Pharmacol 5(1):11–20
Inoue H, Lin L, Lee X, Shao Z, Mendes S, Snodgrass-Belt P, Sweigard H, Engber T et al (2007) Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. Proc Natl Acad Sci U S A 104(36):14430–14435. https://doi.org/10.1073/pnas.0700901104
Oda K, Matsuoka Y, Funahashi A, Kitano H (2005) A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1:2005 0010. https://doi.org/10.1038/msb4100014
Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O'Rourke DM, Holland EC, Garcia-Verdugo JM, Roy NS et al (2010) Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neuro-Oncol 97(3):323–337. https://doi.org/10.1007/s11060-009-0035-x
Li X, Xiao Z, Han J, Chen L, Xiao H, Ma F, Hou X, Li X et al (2013) Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials 34(21):5107–5116. https://doi.org/10.1016/j.biomaterials.2013.03.062
Wang J, Yu RK (2013) Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc Natl Acad Sci U S A 110(47):19137–19142. https://doi.org/10.1073/pnas.1307224110
Novozhilova E, Englund-Johansson U, Kale A, Jiao Y, Olivius P (2015) Effects of ROCK inhibitor Y27632 and EGFR inhibitor PD168393 on human neural precursors co-cultured with rat auditory brainstem explant. Neuroscience 287:43–54. https://doi.org/10.1016/j.neuroscience.2014.12.009
Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W et al (2010) The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials 31(35):9212–9220. https://doi.org/10.1016/j.biomaterials.2010.08.040
Douglas MR, Morrison KC, Jacques SJ, Leadbeater WE, Gonzalez AM, Berry M, Logan A, Ahmed Z (2009) Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain 132(Pt 11):3102–3121. https://doi.org/10.1093/brain/awp240
Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N et al (2004) LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7(3):221–228. https://doi.org/10.1038/nn1188
Lu Y, Liu X, Zhou J, Huang A, Zhou J, He C (2013) TROY interacts with Rho guanine nucleotide dissociation inhibitor alpha (RhoGDIalpha) to mediate Nogo-induced inhibition of neurite outgrowth. J Biol Chem 288(47):34276–34286. https://doi.org/10.1074/jbc.M113.519744
Sharma K, Selzer ME, Li S (2012) Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol 237(2):370–378. https://doi.org/10.1016/j.expneurol.2012.07.009
Yuan YM, He C (2013) The glial scar in spinal cord injury and repair. Neurosci Bull 29(4):421–435. https://doi.org/10.1007/s12264-013-1358-3
Dyck SM, Karimi-Abdolrezaee S (2015) Chondroitin sulfate proteoglycans_ Key modulators in the developing and 3 pathologic central nervous system. Exp Neurol 269:169–187. https://doi.org/10.1016/j.expneurol.2015.04.006
Mckillop WM, Dragan M, Schedl A, Brown AA (2013) Conditional Sox9 ablation reduces chondroitin sulfate proteoglycan levels and improves motor function following spinal cord injury. Glia 61:164–177. https://doi.org/10.1002/glia.22424
Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP et al (2015) Modulation of the proteoglycan receptor PTPs promotes recovery after spinal cord injury. Nature 518(7539):404–408. https://doi.org/10.1038/nature13974
Mi S, Pepinsky RB, Cadavid D (2013) Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs 27(7):493–503. https://doi.org/10.1007/s40263-013-0068-8
Funding
This work was financially supported by the National Natural Science Foundation of China, No. 81371366 (to HFW); the Characteristic Innovation Project of Colleges and Universities in Guangdong Province, No. 2018KTSCX075 (to HFW); the Key Project of Social Development of Dongguan of China, No. 20185071521640 (to HFW); College Students’ Science and Technology Innovation Training Project, No. 201810571058, No. GDMU2018024, No. GDMU2018056, No. GDMU2018061 (to HFW); College Students’ Innovative Experimental Project in Guangdong Medical University, No. ZZDS001 (to HFW); and College Students’ Science and Technology Innovation Cultivation Project in Guangdong, No. pdjh2019b0217 (to HFW).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants performed by any of the authors.
Ethical Approval
All procedures using laboratory animals were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and approved by the Animal Care and Use Committee of Guangdong Medical University at which the studies were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Ding, L., Wang, Y. et al. MiR-615 Regulates NSC Differentiation In Vitro and Contributes to Spinal Cord Injury Repair by Targeting LINGO-1. Mol Neurobiol 57, 3057–3074 (2020). https://doi.org/10.1007/s12035-020-01936-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01936-z