Abstract
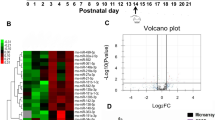
An initial precipitating injury in the brain, such as after status epilepticus (SE), evolves into chronic temporal lobe epilepsy (TLE). We investigated changes in the miRNA composition of extracellular vesicles (EVs) in the forebrain after the establishment of SE-induced chronic TLE. We induced SE in young Fischer 344 rats through graded intraperitoneal injections of kainic acid, which resulted in consistent spontaneous recurrent seizures at ~ 3 months post-SE. We isolated EVs from the entire forebrain of chronically epileptic rats and age-matched naïve control animals through an ultracentrifugation method and performed miRNA-sequencing studies to discern changes in the miRNA composition of forebrain-derived EVs in chronic epilepsy. EVs from both naïve and epileptic forebrains displayed spherical or cup-shaped morphology, a comparable size range, and CD63 expression but lacked the expression of a deep cellular marker GM130. However, miRNA-sequencing studies suggested downregulation of 3 miRNAs (miR-187-5p, miR-346, and miR-331-3p) and upregulation of 4 miRNAs (miR-490-5p, miR-376b-3p, miR-493-5p, and miR-124-5p) in EVs from epileptic forebrains with fold changes ranging from 1.5 to 2.4 (p < 0.0006; FDR < 0.05). By using geNorm and Normfinder software, we identified miR-487 and miR-221 as the best combination of reference genes for measurement of altered miRNAs found in the epileptic forebrain through qRT-PCR studies. The validation revealed that only miR-346 and miR-331-3p were significantly downregulated in EVs from the epileptic forebrain. The enrichment pathway analysis of these miRNAs showed an overrepresentation of signaling pathways that are linked to molecular mechanisms underlying chronic epilepsy, including GABA-ergic (miR-346 targets) and mTOR (miR-331-3p targets) systems. Thus, the packaging of two miRNAs into EVs in neural cells is considerably altered in chronic epilepsy. Functional studies on these two miRNAs may uncover their role in the pathophysiology and treatment of TLE.






Similar content being viewed by others
References
Devinsky O, Vezzani A, O’Brien TJ et al (2018) Epilepsy. Nat Rev Dis Primers 4:18024. https://doi.org/10.1038/nrdp.2018.24
Fisher PD, Sperber EF, Moshé SL (1998) Hippocampal sclerosis revisited. Brain Dev 20:563–573. https://doi.org/10.1016/S0387-7604(98)00069-2
Shetty AK, Zaman V, Hattiangady B (2005) Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci 25:8391–8401. https://doi.org/10.1523/JNEUROSCI.1538-05.2005
Rao MS, Hattiangady B, Reddy DS, Shetty AK (2006) Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res 83:1088–1105. https://doi.org/10.1002/jnr.20802
Hattiangady B, Kuruba R, Shetty AK (2011) Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis 2:1–17
Shetty AK (2002) Entorhinal axons exhibit sprouting in CA1 subfield of the adult hippocampus in a rat model of temporal lobe epilepsy. Hippocampus 12:534–542. https://doi.org/10.1002/hipo.10031
Hattiangady B, Rao MS, Shetty AK (2004) Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis 17:473–490. https://doi.org/10.1016/j.nbd.2004.08.008
Rao MS, Hattiangady B (2008) Shetty AK (2008) Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 18(9):931–944. https://doi.org/10.1002/hipo.20449
Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK (2008) Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav 12:410–418. https://doi.org/10.1016/j.yebeh.2007.11.012
Shetty AK, Turner DA (1999) Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol 414(2):238–254
Hattiangady B, Shetty AK (2010) Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus 20:97–112. https://doi.org/10.1002/hipo.20594
Shetty AK, Upadhya D (2016) GABA-ergic cell therapy for epilepsy: advances, limitations and challenges. Neurosci Biobehav Rev 62:35–47. https://doi.org/10.1016/j.neubiorev.2015.12.014
Shetty AK (2014) Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav 38:117–124. https://doi.org/10.1016/j.yebeh.2013.12.001
Hattiangady B, Shetty AK (2012) Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl Med 9:696–708. https://doi.org/10.5966/sctm.2012-0050
Upadhya D, Hattiangady B, Castro OW, Shuai B, Kodali M, Attaluri S, Bates A, Dong Y et al (2019) Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci U S A 116:287–296. https://doi.org/10.1073/pnas.1814185115
Elliott RC, Miles MF, Lowenstein DH (2003) Overlapping microarray profiles of dentate gyrus gene expression during development- and epilepsy-associated neurogenesis and axon outgrowth. J Neurosci 23:2218–2227
Lukasiuk K, Kontula L, Pitkänen A (2003) cDNA profiling of epileptogenesis in the rat brain. Eur J Neurosci 17:271–279
Hunsberger JG, Bennett AH, Selvanayagam E et al (2005) Gene profiling the response to kainic acid induced seizures. Brain Res Mol Brain Res 141:95–112. https://doi.org/10.1016/j.molbrainres.2005.08.005
Gorter JA, van Vliet EA, Aronica E et al (2006) Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci 26:11083–11110. https://doi.org/10.1523/JNEUROSCI.2766-06.2006
Jimenez-Mateos EM, Hatazaki S, Johnson MB, Bellver-Estelles C, Mouri G, Bonner C, Prehn JH, Meller R et al (2008) Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiology of Disease 32:442–453. https://doi.org/10.1016/j.nbd.2008.08.008
Romcy-Pereira RN, Gitaí DLG, Gitaí LLG et al (2008) Genes e epilepsia II: expressão gênica diferencial. Rev Assoc Méd Bras 54. https://doi.org/10.1590/S0104-42302008000500022
Laurén HB, Lopez-Picon FR, Brandt AM, Rios-Rojas CJ, Holopainen IE (2010) Transcriptome analysis of the hippocampal CA1 pyramidal cell region after kainic acid-induced status epilepticus in juvenile rats. PLoS One 5:e10733. https://doi.org/10.1371/journal.pone.0010733
de Araújo MA, Marques TEBS, Octacílio-Silva S et al (2016) Identification of microRNAs with dysregulated expression in status epilepticus induced epileptogenesis. PLoS One 11:e0163855. https://doi.org/10.1371/journal.pone.0163855
Kinjo ER, Higa GSV, Santos BA, de Sousa E, Damico MV, Walter LT, Morya E, Valle AC et al (2016) Pilocarpine-induced seizures trigger differential regulation of microRNA-stability related genes in rat hippocampal neurons. Sci Rep 6:6–13. https://doi.org/10.1038/srep20969
Gitaí DLG, Fachin AL, Mello SS, Elias CF, Bittencourt JC, Leite JP, Passos GA, Garcia-Cairasco N et al (2011) The non-coding RNA BC1 is down-regulated in the hippocampus of Wistar Audiogenic Rat (WAR) strain after audiogenic kindling. Brain Res 1367:114–121. https://doi.org/10.1016/j.brainres.2010.10.069
Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan R, Tanaka K, Mouri G, Sano T, O'Tuathaigh C et al (2012) Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18:1087–1094. https://doi.org/10.1038/nm.2834
Lee DY, Moon J, Lee S-T, Jung KH, Park DK, Yoo JS, Sunwoo JS, Byun JI et al (2015) Dysregulation of long non-coding RNAs in mouse models of localization-related epilepsy. Biochem Biophys Res Commun 462:433–440. https://doi.org/10.1016/j.bbrc.2015.04.149
Miller-Delaney SFC, Bryan K, Das S, McKiernan R, Bray IM, Reynolds JP, Gwinn R, Stallings RL et al (2015) Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 138:616–631. https://doi.org/10.1093/brain/awu373
Kiese K, Jablonski J, Hackenbracht J, Wrosch JK, Groemer TW, Kornhuber J, Blümcke I, Kobow K (2017) Epigenetic control of epilepsy target genes contributes to a cellular memory of epileptogenesis in cultured rat hippocampal neurons. Acta Neuropathol Commun 5:79. https://doi.org/10.1186/s40478-017-0485-x
Hauser RM, Henshall DC, Lubin FD (2018) The epigenetics of epilepsy and its progression. Neuroscientist 24:186–200. https://doi.org/10.1177/1073858417705840
Henshall DC (2018) Epigenetic changes in status epilepticus. Epilepsia 59(Suppl 2):82–86. https://doi.org/10.1111/epi.14502
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20. https://doi.org/10.1016/j.cell.2004.12.035
Fiorenza A, Lopez-Atalaya JP, Rovira V, Scandaglia M, Geijo-Barrientos E, Barco A (2016) Blocking miRNA biogenesis in adult forebrain neurons enhances seizure susceptibility, fear memory, and food intake by increasing neuronal responsiveness. Cereb Cortex 26:1619–1633. https://doi.org/10.1093/cercor/bhu332
Risbud RM, Porter BE (2013) Changes in microRNA expression in the whole hippocampus and hippocampal synaptoneurosome fraction following pilocarpine induced status epilepticus. PLoS One 8:e53464. https://doi.org/10.1371/journal.pone.0053464
Henshall DC, Hamer HM, Pasterkamp RJ, Goldstein DB, Kjems J, Prehn JHM, Schorge S, Lamottke K et al (2016) MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol 15:1368–1376. https://doi.org/10.1016/S1474-4422(16)30246-0
Surges R, Kretschmann A, Abnaof K, van Rikxoort M, Ridder K, Fröhlich H, Danis B, Kaminski RM et al (2016) Changes in serum miRNAs following generalized convulsive seizures in human mesial temporal lobe epilepsy. Biochem Biophys Res Commun 481:13–18. https://doi.org/10.1016/j.bbrc.2016.11.029
Raoof R, Jimenez-Mateos EM, Bauer S, Tackenberg B, Rosenow F, Lang J, Onugoren MD, Hamer H et al (2017) Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Sci Rep 7:3328. https://doi.org/10.1038/s41598-017-02969-6
Raoof R, Bauer S, El Naggar H et al (2018) Dual-center, dual-platform microRNA profiling identifies potential plasma biomarkers of adult temporal lobe epilepsy. EBioMedicine 38:127–141. https://doi.org/10.1016/j.ebiom.2018.10.068
Bayraktar R, Van Roosbroeck K, Calin GA (2017) Cell-to-cell communication: microRNAs as hormones. Mol Oncol 11:1673–1686. https://doi.org/10.1002/1878-0261.12144
Paolicelli RC, Bergamini G, Rajendran L (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157. https://doi.org/10.1016/j.neuroscience.2018.04.003
Goetzl L, Merabova N, Darbinian N, Martirosyan D, Poletto E, Fugarolas K, Menkiti O (2018) Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol 5:4–10. https://doi.org/10.1002/acn3.499
Vogel A, Upadhya R, Shetty AK (2018) Neural stem cell derived extracellular vesicles: attributes and prospects for treating neurodegenerative disorders. EBioMedicine 38:273–282. https://doi.org/10.1016/j.ebiom.2018.11.026
Liu X, Yuan W, Yang L, Li J, Cai J (2019) miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res 12:75–83. https://doi.org/10.1007/s12265-017-9784-7
Manna I, Iaccino E, Dattilo V, Barone S, Vecchio E, Mimmi S, Filippelli E, Demonte G et al (2018) Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. The FASEB Journal 32:4241–4246. https://doi.org/10.1096/fj.201701533R
van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64:676–705. https://doi.org/10.1124/pr.112.005983
Willms E, Cabañas C, Mäger I, Wood MJA, Vader P (2018) Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Frontiers in Immunology 9:738. https://doi.org/10.3389/fimmu.2018.00738
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/jcb.201211138
Isola A, Chen S (2016) Exosomes: the messengers of health and disease. Current Neuropharmacology 15:157–165
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. https://doi.org/10.1080/20013078.2018.1535750
Kawikova I, Askenase PW (2015) Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res 1617:63–71. https://doi.org/10.1016/j.brainres.2014.09.070
Guay C, Regazzi R (2017) Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 19(Suppl 1):137–146. https://doi.org/10.1111/dom.13027
Blandford SN, Galloway DA, Moore CS (2018) The roles of extracellular vesicle microRNAs in the central nervous system. Glia 66:2267–2278. https://doi.org/10.1002/glia.23445
Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J (2018) The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep 8:1419. https://doi.org/10.1038/s41598-018-19581-x
Tan L, Wu H, Liu Y, Zhao M, Li D, Lu Q (2016) Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity 49:357–365. https://doi.org/10.1080/08916934.2016.1191477
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, Beadnall H, Barnett MH et al (2017) Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep 7:14293. https://doi.org/10.1038/s41598-017-14301-3
Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B (2017) Exosomes, an unmasked culprit in neurodegenerative diseases. Frontiers in Neuroscience 11:26. https://doi.org/10.3389/fnins.2017.00026
Tomasetti M, Lee W, Santarelli L, Neuzil J (2017) Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Experimental & Molecular Medicine 49:e285–e285. https://doi.org/10.1038/emm.2016.153
Yan S, Zhang H, Xie W et al (2017) Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 8:4136–4146. https://doi.org/10.18632/oncotarget.13744
Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK (2017) Intranasal MSC-derived A1-exosomes ease inflammation and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA 114:E3536–E3545. https://doi.org/10.1073/pnas.1703920114
Upadhya D, Shetty AK (2019) Promise of extracellular vesicles for diagnosis and treatment of epilepsy. Epilepsy Behav, in press https://doi.org/10.1016/j.yebeh.2019.106499
Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE (1998) Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res 31:73–84
Bertoglio D, Amhaoul H, Van Eetveldt A et al (2017) Kainic acid-induced post-status epilepticus models of temporal lobe epilepsy with diverging seizure phenotype and neuropathology. Front Neurol 8:588. https://doi.org/10.3389/fneur.2017.00588
Upadhya D, Kodali M, Gitai D, Castro OW, Zanirati G, Upadhya R, Attaluri S, Mitra E, Shuai B, Hattiangady B, Shetty AK (2019) A model of temporal lobe epilepsy presenting constantly rhythmic and robust spontaneous seizures, co-morbidities and hippocampal neuropathology. Aging and Dis, in press.
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Li P, Kaslan M, Lee SH, Yao J, Gao Z (2017) Progress in exosome isolation techniques. Theranostics 7:789–804. https://doi.org/10.7150/thno.18133
Vella LJ, Scicluna BJ, Cheng L, Bawden EG, Masters CL, Ang CS, Willamson N, McLean C et al (2017) A rigorous method to enrich for exosomes from brain tissue. J Extracell Vesicles 6:1348885. https://doi.org/10.1080/20013078.2017.1348885
Madhu LN, Attaluri S, Kodali M et al (2019) Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immun 81:430-443 https://doi.org/10.1016/j.bbi.2019.06.040
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43:W460–W466. https://doi.org/10.1093/nar/gkv403
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K et al (2000) Gene ontology: tool for the unification of biology. Nature Genetics 25:25–29
Kanehisa M (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research 28:27–30
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M (2016) KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44:D457–D462. https://doi.org/10.1093/nar/gkv1070
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2017) KEGG: new perspectives on genomes, p3thways, diseases and drugs. Nucleic Acids Res 45:D353–D361. https://doi.org/10.1093/nar/gkw1092
The Gene Ontology Consortium, The Gene Ontology Consortium (2017) Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Research 45:D331–D338. https://doi.org/10.1093/nar/gkw1108
Bleazard T, Lamb JA, Griffiths-Jones S (2015) Bias in microRNA functional enrichment analysis. Bioinformatics 31:1592–1598. https://doi.org/10.1093/bioinformatics/btv023
Nakamura N (2010) Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J Pharmacol Sci 112:255–264
Lötvall J, Hill AF, Hochberg F, Buzás EI, di Vizio D, Gardiner C, Gho YS, Kurochkin IV et al (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913. https://doi.org/10.3402/jev.v3.26913
Ashhab MU, Omran A, Kong H, Gan N, He F, Peng J, Yin F (2013) Expressions of tumor necrosis factor alpha and microRNA-155 in immature rat model of status epilepticus and children with mesial temporal lobe epilepsy. J Mol Neurosci 51:950–958. https://doi.org/10.1007/s12031-013-0013-9
Henshall DC (2013) Antagomirs and microRNA in status epilepticus. Epilepsia 54(Suppl 6):17–19. https://doi.org/10.1111/epi.12267
Henshall DC (2013) MicroRNAs in the pathophysiology and treatment of status epilepticus. Front Mol Neurosci 6:37. https://doi.org/10.3389/fnmol.2013.00037
Marques TEBS, de Mendonça LR, Pereira MG, de Andrade TG, Garcia-Cairasco N, Paçó-Larson ML, Gitaí DL (2013) Validation of suitable reference genes for expression studies in different pilocarpine-induced models of mesial temporal lobe epilepsy. PLoS One 8:e71892. https://doi.org/10.1371/journal.pone.0071892
de Araújo MA, Marques TEBS, Taniele-Silva J, Souza FM, de Andrade TG, Garcia-Cairasco N, Paçó-Larson ML, Gitaí DL (2014) Identification of endogenous reference genes for the analysis of microRNA expression in the hippocampus of the pilocarpine-induced model of mesial temporal lobe epilepsy. PLoS One 9:e100529. https://doi.org/10.1371/journal.pone.0100529
da Silva Santos EA, TEBS M, de Carvalho Matos H et al (2015) Diurnal variation has effect on differential gene expression analysis in the hippocampus of the pilocarpine-induced model of mesial temporal lobe epilepsy. PLoS One 10:e0141121. https://doi.org/10.1371/journal.pone.0141121
Born JPL, Matos CH, de Araujo MA et al (2017) Using postmortem hippocampi tissue can interfere with differential gene expression analysis of the epileptogenic process. PLoS One 12:e0182765. https://doi.org/10.1371/journal.pone.0182765
Brindley E, Hill TDM, Henshall DC (2019) MicroRNAs as biomarkers and treatment targets in status epilepticus. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2019.04.025
Schouten M, Fratantoni SA, Hubens CJ, Piersma SR, Pham TV, Bielefeld P, Voskuyl RA, Lucassen PJ et al (2015) MicroRNA-124 and -137 cooperativity controls caspase-3 activity through BCL2L13 in hippocampal neural stem cells. Sci Rep 5:12448. https://doi.org/10.1038/srep12448
Kretschmann A, Danis B, Andonovic L, Abnaof K, van Rikxoort M, Siegel F, Mazzuferi M, Godard P et al (2015) Different microRNA profiles in chronic epilepsy versus acute seizure mouse models. J Mol Neurosci 55:466–479. https://doi.org/10.1007/s12031-014-0368-6
Lim JS, Kim W-I, Kang H-C, Kim SH, Park AH, Park EK, Cho YW, Kim S et al (2015) Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21:395–400. https://doi.org/10.1038/nm.3824
Treiman DM (2001) GABAergic mechanisms in epilepsy. Epilepsia 42:8–12
Meng X-F, Yu J-T, Song J-H, Chi S, Tan L (2013) Role of the mTOR signaling pathway in epilepsy. J Neurol Sci 332:4–15. https://doi.org/10.1016/j.jns.2013.05.029
Baulac S (2016) mTOR signaling pathway genes in focal epilepsies. Prog Brain Res 226:61–79. https://doi.org/10.1016/bs.pbr.2016.04.013
Van Zandt MA, Naegele JR (2017) GABAergic synapse dysfunction and repair in temporal lobe epilepsy. In: Heinbockel T (ed) Synaptic plasticity. InTech. https://doi.org/10.5772/67218
Gorlewicz A, Kaczmarek L (2018) Pathophysiology of trans-synaptic adhesion molecules: implications for epilepsy. Frontiers in Cell and Developmental Biology 6:119. https://doi.org/10.3389/fcell.2018.00119
Somera-Molina KC, Robin B, Somera CA, Anderson C, Stine C, Koh S, Behanna HA, van Eldik L et al (2007) Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia 48:1785–1800. https://doi.org/10.1111/j.1528-1167.2007.01135.x
Yang L, Li F, Ge W, Mi C, Wang R, Sun R (2010) Protective effects of naloxone in two-hit seizure model. Epilepsia 51:344–353. https://doi.org/10.1111/j.1528-1167.2009.02250.x
Feng S, Ma S, Jia C, Su Y, Yang S, Zhou K, Liu Y, Cheng J et al (2016) Sonic hedgehog is a regulator of extracellular glutamate levels and epilepsy. EMBO Rep 17:682–694. https://doi.org/10.15252/embr.201541569
Dubey D, McRae PA, Rankin-Gee EK, Baranov E, Wandrey L, Rogers S, Porter BE (2017) Increased metalloproteinase activity in the hippocampus following status epilepticus. Epilepsy Res 132:50–58. https://doi.org/10.1016/j.eplepsyres.2017.02.021
Lai K, Kaspar BK, Gage FH, Schaffer DV (2003) Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 6:21–27. https://doi.org/10.1038/nn983
Hester MS, Danzer SC (2013) Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci 33:8926–8936. https://doi.org/10.1523/JNEUROSCI.5161-12.2013
Yao PJ, Petralia RS, Mattson MP (2016) Sonic hedgehog signaling and hippocampal neuroplasticity. Trends Neurosci 39:840–850. https://doi.org/10.1016/j.tins.2016.10.001
Ikonomidou C (2014) Matrix metalloproteinases and epileptogenesis. Mol Cell Pediatr 1:6. https://doi.org/10.1186/s40348-014-0006-y
Rempe RG, Hartz AMS, Bauer B (2016) Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab 36:1481–1507. https://doi.org/10.1177/0271678X16655551
Sakatani S, Seto-Ohshima A, Shinohara Y, Yamamoto Y, Yamamoto H, Itohara S, Hirase H (2008) Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo. J Neurosci 28:10928–10936. https://doi.org/10.1523/JNEUROSCI.3693-08.2008
Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I, Donato R (2010) (2010) The many faces of S100B protein: when an extracellular factor inactivates its own receptor and activates another one. Ital J Anat Embryol. 115(1-2):147–151
Lu C, Li J, Sun W, Feng L, Li L, Liu A, Li J, Mao W et al (2010) Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: a case–control study. Neurosci Lett 484:139–142. https://doi.org/10.1016/j.neulet.2010.08.036
Calik M, Abuhandan M, Sonmezler A, Kandemır H, Oz I, Taskin A, Selek S, Iscan A (2013) Elevated serum S-100B levels in children with temporal lobe epilepsy. Seizure 22:99–102. https://doi.org/10.1016/j.seizure.2012.10.012
Meguid NA, Samir H, Bjørklund G, Anwar M, Hashish A, Koura F, Chirumbolo S, Hashem S et al (2018) Altered S100 calcium-binding protein B and matrix metallopeptidase 9 as biomarkers of mesial temporal lobe epilepsy with hippocampus sclerosis. J Mol Neurosci 66:482–491. https://doi.org/10.1007/s12031-018-1164-5
Funding
This work was supported by grants from the Department of Defense (W81XWH-14-1-0558 to A.K.S.), the State of Texas (Emerging Technology Fund to A.K.S.), and the Department of Veterans Affairs (Merit Award I01BX000883 and BLR&D Research Career Scientist award 1IK6BX003612 to A.K.S.). Daniel Gitai was supported by a Visiting Scientist Award from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Government of Brazil (D.L.G. Gitai).
Author information
Authors and Affiliations
Contributions
Conception: DLGG and AKS; research design: DLGG, RU, MK, and AKS; collection of data: DLGG, YDRS, RU, MK, and LNM; data analyses and interpretation: DLGG, YDRS, and AKS; preparation of figures: DLGG, YDRS, LNM, and AKS; manuscript writing: DLGG and AKS.
Corresponding authors
Ethics declarations
The Animal Care and Use Committee of the Texas A&M University approved all animal experiments performed in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gitaí, D.L.G., dos Santos, Y.D.R., Upadhya, R. et al. Extracellular Vesicles in the Forebrain Display Reduced miR-346 and miR-331-3p in a Rat Model of Chronic Temporal Lobe Epilepsy. Mol Neurobiol 57, 1674–1687 (2020). https://doi.org/10.1007/s12035-019-01797-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01797-1