Abstract
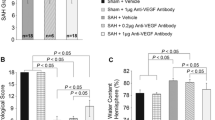
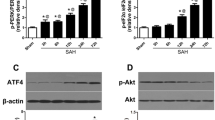
Neuronal apoptosis is a common and critical pathology following subarachnoid hemorrhage (SAH). We investigated the anti-apoptotic property of fibroblast growth factor (FGF)-2 after SAH in rats. A total of 289 rats underwent endovascular perforation to induce SAH or sham operation. Three dosages (3, 9, or 27 μg) of recombinant FGF-2 (rFGF-2) or vehicle was administered intranasally to rats 30 min after SAH induction. The pan-FGF receptor (FGFR) inhibitor PD173074 or vehicle was administered intracerebroventricularly (i.c.v.) 1 h before modeling, in addition to rFGF-2 treatment. Small interfering ribonucleic acid (siRNA) for FGFR1 and FGFR3 or scrambled siRNA was administered i.c.v. 48 h before SAH induction in addition to rFGF-2 treatment. Anti-FGF-2 neutralizing antibody or normal mouse immunoglobulin G (IgG) was administered i.c.v. 1 h before SAH model. Neurobehavioral tests, SAH severity, brain water content, immunofluorescence, Fluoro-Jade C, TUNEL staining, and western blot were evaluated. The expression of FGF-2, FGFR1, and FGFR3 increased after SAH. FGFR1 and FGFR3 were expressed in the neurons. Nine micrograms of FGF-2 alleviated neurological impairments, brain edema, and neuronal apoptosis following SAH. A rFGF-2 treatment improved motor skill learning and spatial memory and increased the number of surviving neurons postinjury to 28 days after SAH. PD173074 abolished the anti-apoptotic effects of rFGF-2 via suppression of the expression of PI3k, phosphorylated Akt (p-Akt), and Bcl-2 leading to enhancement of the expression of Bax. FGFR3 siRNA worsened neurobehavioral function and suppressed the expression of PI3k, p-Akt, and Bcl-2 rather than FGFR1 siRNA in SAH rats treated with rFGF-2. Anti-FGF-2 neutralizing antibody suppressed the expression of PI3k and p-Akt after SAH. FGF-2 may be a promising therapy to reduce post-SAH neuronal apoptosis via activation of the FGFR3/PI3k/Akt signaling pathway.











Similar content being viewed by others
Abbreviations
- BWC :
-
brain water content
- CA :
-
cornu ammonis
- CNS :
-
central nervous system
- DG :
-
dentate gyrus
- DMSO :
-
dimethyl sulfoxide
- EBI :
-
early brain injury
- ERK :
-
extracellular signal-regulated kinase
- FGF :
-
fibroblast growth factor
- FGFR :
-
fibroblast growth factor receptor
- FJC :
-
Fluoro-Jade C
- GRB2 :
-
growth factor receptor-bound protein 2
- i.c.v.:
-
intracerebroventricular
- IgG :
-
immunoglobulin G
- IHC :
-
immunohistochemistry
- Nab :
-
neutralizing antibody
- NeuN :
-
neuron-specific nuclear protein
- p-Akt :
-
phosphorylated Akt
- PBS :
-
phosphate-buffered saline
- p-FGFR :
-
phosphorylated fibroblast growth factor receptor
- rFGF-2 :
-
recombinant fibroblast growth factor-2
- rpm :
-
revolutions per minute
- SAH :
-
subarachnoid hemorrhage
- Scr siRNA :
-
scrambled small interfering ribonucleic acid
- siRNA :
-
small interfering ribonucleic acid
- SOS :
-
son of sevenless
- TUNEL :
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WB :
-
western blot
References
Okada T, Suzuki H (2017) Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res 12:193
Prunell GF, Svendgaard NA, Alkass K, Mathiesen T (2005) Delayed cell death related to acute cerebral blood flow changes following subarachnoid hemorrhage in the rat brain. J Neurosurg 102:1046–1054
Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li T, Shi JX, Zhang X (2014) Resveratrol prevents neuronal apoptosis in an early brain injury model. J Surg Res 189:159–165
Noda M, Takii K, Parajuli B, Kawanokuchi J, Sonobe Y, Takeuchi H et al (2014) FGF-2 released from degenerating neurons exerts microglial-induced neuroprotection via FGFR3-ERK signaling pathway. J Neuroinflamm 11:1–11
Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang X, Yan Y, Fu X et al (2012) BFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett 212:137–146
Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH, Johnson NR, Wei X, Chen DQ et al (2016) bFGF protects against blood-brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol 53:7298–7311
Huang B, Krafft PR, Ma Q, Rolland WB, Caner B, Lekic T, Manaenko A, le M et al (2012) Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis 46:204–214
Tang C, Shan Y, Hu Y, Fang Z, Tong Y, Chen M, Wei X, Fu X et al (2017) FGF2 attenuates neural cell death via suppressing autophagy after rat mild traumatic brain injury. Stem Cells Int 2017:1–14. https://doi.org/10.1155/2017/2923182
Reilly JF, Kumari VG (1996) Alterations in fibroblast growth factor receptor expression following brain injury. Exp Neurol 140:139–150
Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F et al (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem 271:15292–15297
Enkhjargal B, McBride DW, Manaenko A, Reis C, Sakai Y, Tang J et al (2017) Intranasal administration of vitamin D attenuates blood–brain barrier disruption through endogenous upregulation of osteopontin and activation of CD44/P-gp glycosylation signaling after subarachnoid hemorrhage in rats. J Cerebr Blood F and Met 37:2555–2566
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Meth 167:327–334
Mo J, Enkhjargal B, Travis ZD, Zhou K, Wu P, Zhang G, Zhu Q, Zhang T et al (2019) AVE 0991 attenuates oxidative stress and neuronal apoptosis via Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats. Redox Biol 20:75–86
Feng C, Zhang C, Shao X, Liu Q, Qian Y, Feng L, Chen J, Zha Y et al (2012) Enhancement of nose-to-brain delivery of basic fibroblast growth factor for improving rat memory impairments induced by co-injection of β-amyloid and ibotenic acid into the bilateral hippocampus. Int J Pharm 423:226–234
Zhou K, Enkhjargal B, Xie Z, Sun C, Wu L, Malaguit J, Chen S, Tang J et al (2018) Dihydrolipoic acid inhibits lysosomal rupture and NLRP3 through lysosome-associated membrane Protein-1/Calcium/Calmodulin-Dependent Protein Kinase II/TAK1 pathways after subarachnoid hemorrhage in rat. Stroke 49:175–183
Zappaterra MW, LaMantia AS, Walsh CA, Lehtinen MK (2013) Isolation of cerebrospinal fluid from rodent embryos for use with dissected cerebral cortical explants. J Vis Exp 73:e50333.
Westerhout J, Ploeger B, Smeets J, Danhof M, de Lange ECM (2012) Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats. The AAPS J 14:543–553
Wen TC, Matsuda S, Yoshimura H, Aburaya J, Kushihata F, Sakanaka M (1995) Protective effect of basic fibroblast growth factor-heparin and neurotoxic effect of platelet factor 4 on ischemic neuronal loss and learning disability in gerbils. Neuroscience 65:513–521
Okada T, Kawakita F, Nishikawa H, Nakano F, Liu L, Suzuki H (2019) Selective toll-like receptor 4 antagonists prevent acute blood-brain barrier disruption after subarachnoid hemorrhage in mice. Mol Neurobiol 56:976–985
Xie Z, Enkhjargal B, Wu L, Zhou K, Sun C, Hu X, Gospodarev V, Tang J et al (2018) Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 128:142–151
Bromley-Brits K, Deng Y, Song W (2011) Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp 53:e2920
Kanai M, Go M, Tsunekawa S, Podolsky DK (1997) Signal transduction pathway of human fibroblast growth factor receptor 3. J Biol Chem 272:6621–6628
Moisa H, Palade C, Nica D, Savu R, Ciurea A (2013) Interference of apoptosis in the pathophysiology of subarachnoid hemorrhage. Asian J Neurosurg 8:106–111
Endo H, Nito C, Kamada H, Yu F, Chan PH (2007) Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cerebr Blood F Met 27:975–982
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T et al (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429–441
Logan A, Frautschy SA, Gonzalez AM, Baird A (1992) A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci 12:3828–3837
Rowntree S, Kolb B (1997) Blockade of basic fibroblast growth factor retards recovery from motor cortex injury in rats. Eur J Neurosci 9:2432–2441
Fagan AM, Suhr ST, Lucidi-Phillipi CA, Peterson DA, Holtzman DM, Gage FH (1997) Endogenous FGF-2 is important for cholinergic sprouting in the denervated hippocampus. J Neurosci 17:2499–2511
Zhu Q, Enkhjargal B, Huang L, Zhang T, Sun C, Xie Z et al (2018) Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-ΚB pathway after subarachnoid hemorrhage in rats. J Neuroinflamm. 15:1–13
Kim J, Gale K, Kondratyev A (2010) Effects of repeated minimal electroshock seizures on NGF, BDNF and FGF-2 protein in the rat brain during postnatal development. Int J Dev Neurosci 28:227–232
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97:14–37
Marbacher S, Neuschmelting V, Andereggen L, Widmer HR, von Gunten M, Takala J, Jakob SM, Fandino J (2014) Early brain injury linearly correlates with reduction in cerebral perfusion pressure during the hyperacute phase of subarachnoid hemorrhage. Intensive Care Med Exp 2:30. https://doi.org/10.1186/s40635-014-0030-1
Acknowledgments
The authors acknowledge the technical assistance provided by Desislava Doycheva, Jerry Flores, Marcin Gamdzyk, Helen Huang, Prativa Sherchan, Orhan Altay, Weilin Xu, Yuchun Zuo, Ganz Zuo, and Jun Peng.
Funding
This article is supported partially by grants from the National Institutes of Health (NS081740 and NS082184) to Dr. Zhang.
Author information
Authors and Affiliations
Contributions
TO, BE, SH, JT, and JHZ were involved in the study design. TO, BE, and JHZ were involved in data interpretation and writing of the manuscript; TO performed the majority of the laboratory work and contributed to the analysis of data; BE was responsible for the animal model; ZDT was involved in manuscript editing. UO was responsible for the rotarod and water maze test; ZDT was involved in manuscript editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The Institutional Animal Care and Use Committee (IACUC) of Loma Linda University approved the study protocol, and all experiments were conducted in accordance with the NIH Guidelines for Use of Animals in Neuroscience Research. All authors have read and approved the submitted manuscript.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 948 kb)
Rights and permissions
About this article
Cite this article
Okada, T., Enkhjargal, B., Travis, Z.D. et al. FGF-2 Attenuates Neuronal Apoptosis via FGFR3/PI3k/Akt Signaling Pathway After Subarachnoid Hemorrhage. Mol Neurobiol 56, 8203–8219 (2019). https://doi.org/10.1007/s12035-019-01668-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01668-9