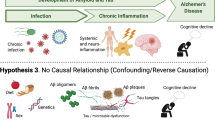
Abstract
Over the past three decades, there has been constant postulation regarding the infectious etiology of Alzheimer disease (AD), which in turn suggests the vital role of various infectious agents in AD-associated inflammatory pathways. Recent findings indicate anti-microbial properties of Aβ, and suggest that Aβ production and deposition in AD might be induced by infectious agents. Several types of spirochetes have been associated to dementia, cortical atrophy, and pathological and biological hallmarks of AD. A significant association between AD spirochetes and other pathogens like HSV-1 and Chlamydia pneumonia has now become well established. In neurons infected by HSV-1 showed Aβ and hyperphosphorylated Tau accumulation. The expression of pro-inflammatory molecules have been found to be enhanced by specific bacterial ligands, and viral and bacterial DNA and RNA, thus activating the immune system. Aβ has now been established as anti-microbial peptide capable of inducing pore formation, thus justifying their infection-mediated accumulation. Thus, a proper combination of anti-inflammatory, anti-viral, and antibiotic therapeutics might potentially prevent the progression of AD. Here, we discussed the potential role of bacterial, fungi, and viral infections in AD causation and progression, and the potential-associated therapies to counter the AD condition.





Similar content being viewed by others
Abbreviations
- ACh:
-
acetylcholine
- ACV:
-
acyclovir
- AD:
-
Alzheimer disease
- Aβ:
-
amyloid-β
- APOE-e4:
-
apolipoprotein E-e4
- BBB:
-
blood brain barrier
- CNS:
-
central nervous system
- CMV:
-
cytomegalovirus
- HSE:
-
herpes simplex encephalitis
- HSV:
-
herpes simplex virus
- HD:
-
Huntington’s disease
- HHV:
-
human herpesvirus
- IVIG:
-
intravenous immunoglobulin
- IAV:
-
influenza A virus
- MS:
-
multiple sclerosis
- NDDs:
-
neurodegenerative disorders
- NFTs:
-
neurofibrillary tangles
- PAMP:
-
pathogen-associated molecular patterns
- PD:
-
Parkinson’s disease
- PBL:
-
peripheral blood leukocytes
- ROS:
-
reactive oxygen species
- VZV:
-
varicella zoster virus
References
Alzheimer’s A (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11:332–384
Ansari SA, Satar R, Perveen A, Ashraf GM (2017) Current opinion in Alzheimer’s disease therapy by nanotechnology-based approaches. Curr Opin Psychiatry 30:128–135
Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK et al (2014) Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets 13:1280–1293
Blocq P, Marinescu G. Sur les lésions et la pathogénie de l'épilepsie dite essentielle S.L.: s.n.; 1892.
Kumar A, Singh A, Ekavali N (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacological reports: PR 67:195–203
Simchowicz T. Histologische Studien über die senile Demenz. Histologische und histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten [Texte imprimé] / herausgegeben von Franz Nissl,... 1911.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580
Gallyas F (1971) Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphologica Academiae Scientiarum Hungaricae 19:1–8
Mawanda F, Wallace R (2013) Can infections cause Alzheimer’s disease? Epidemiol Rev 35:161–180
White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, Hartshorn KL (2014) Alzheimer’s associated β-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One 9:e101364
Lim SL, Rodriguez-Ortiz CJ, Kitazawa M (2015) Infection, systemic inflammation, and Alzheimer’s disease. Microbes and Infection / Institut Pasteur 17:549–556
Hardy J (1997) The Alzheimer family of diseases: many etiologies, one pathogenesis? Proc Natl Acad Sci U S A 94:2095–2097
Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D, Yu ACH (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 8:67–80
Nagy Z (2005) The last neuronal division: a unifying hypothesis for the pathogenesis of Alzheimer’s disease. J Cell Mol Med 9:531–541
Aliev G, Burzynski G, Ashraf GM, Jabir NR, Cacabelos R, Benberin VV, Burzynski SR (2011) Implication of oxidative stress-induced oncogenic signaling pathways as a treatment strategy for neurodegeneration and cancer. In: Systems Biology of Free Radicals and Antioxidants. edited by Laher I. Springer Berlin Heidelberg; pp. 2325–2347.
Aliev G, Priyadarshini M, Reddy VP, Grieg NH, Kaminsky Y, Cacabelos R, Ashraf GM, Jabir NR et al (2014) Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr Med Chem 21:2208–2217
Marx F, Blasko I, Pavelka M, Grubeck-Loebenstein B (1998) The possible role of the immune system in Alzheimer’s disease. Exp Gerontol 33:871–881
Li Y, Tan M-S, Jiang T, Tan L (2014) Microglia in Alzheimer’s disease. Biomed Res Int 2014:437483
Jacobs AH, Tavitian B (2012) Consortium IN. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab 32:1393–1415
Całkosiński I, Dobrzyński M, Całkosińska M, Seweryn E, Bronowicka-Szydełko A, Dzierzba K, Ceremuga I, Gamian A (2009) Characterization of an inflammatory response. Postȩpy Higieny I Medycyny Doświadczalnej (Online) 63:395–408
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol. Series A, Biol Sci Med Sci 69(Suppl 1):S4–S9
Krstic D, Knuesel I (2013) Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 9:25–34
Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science (New York, N.Y.) 217: 408–414, 1982.
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12:383–388
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P et al (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405
McGeer PL, McGeer EG (2002) Local neuroinflammation and the progression of Alzheimer’s disease. J Neurovirol 8:529–538
Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8(112)
Schwab C, McGeer PL (2008) Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimer’s Dis: JAD 13:359–369
Lin WR, Shang D, Wilcock GK, Itzhaki RF (1995) Alzheimer’s disease, herpes simplex virus type 1, cold sores and apolipoprotein E4. Biochem Soc Trans 23:594S
Barichello T, Generoso JS, Goularte JA, Collodel A, Pitcher MR, Simões LR, Quevedo J, Dal-Pizzol F (2015) Does infection-induced immune activation contribute to dementia? Aging Dis 6:342–348
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C et al (2016) Microbes and Alzheimer’s disease. J Alzheimer’s Dis: JAD 51:979–984
Licastro F, Carbone I, Raschi E, Porcellini E (2014) The 21st century epidemic: infections as inductors of neuro-degeneration associated with Alzheimer’s disease. Immunity & Ageing: I & A 11(22):22
Roubaud Baudron C, Varon C, Mégraud F, Salles N (2015) Alzheimer’s disease: the infectious hypothesis. Geriatrie Et Psychologie Neuropsychiatrie Du Vieillissement 13:418–424
Ferrari CC, Tarelli R (2011) Parkinson's disease and systemic inflammation. Parkinson’s Disease 2011:e436813
Nociti V, Frisullo G, Marti A, Luigetti M, Iorio R, Patanella AK, Bianco A, Tonali PA et al (2010) Epstein-Barr virus antibodies in serum and cerebrospinal fluid from multiple sclerosis, chronic inflammatory demyelinating polyradiculoneuropathy and amyotrophic lateral sclerosis. J Neuroimmunol 225:149–152
Maheshwari P, Eslick GD (2015) Bacterial infection and Alzheimer’s disease: a meta-analysis. J Alzheimer's Dis: JAD 43:957–966
Bibi F, Yasir M, Sohrab SS, Azhar EI, Al-Qahtani MH, Abuzenadah AM, Kamal MA, Naseer MI (2014) Link between chronic bacterial inflammation and Alzheimer disease. CNS Neurol Disord Drug Targets (CNS&NDDT) 13:1140–1147
Miklossy J (2015) Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci 7(46)
Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat Inflamm 2013:480739
Miklossy J (2011) Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation 8:90
Nicolson GL (2008) Chronic bacterial and viral infections in neurodegenerative and neurobehavioral diseases. Lab Med 39:291–299
Riviere GR, Riviere KH, Smith KS (2002) Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol 17:113–118
Olsen I, Singhrao SK (2015) Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol 7:29143
Miklossy J (2011) Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13:e30
Abbayya K, Puthanakar NY, Naduwinmani S, Chidambar YS (2015) Association between periodontitis and Alzheimer’s disease. N Am J Med Sci 7:241–246
Miklossy J (1993) Alzheimer’s disease--a spirochetosis? Neuroreport 4:841–848
Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J (2003) Early olfactory involvement in Alzheimer’s disease. The Canadian Journal of Neurological Sciences Le Journal Canadien Des Sciences Neurologiques 30:20–25
MacDonald AB (1988) Concurrent neocortical borreliosis and Alzheimer’s disease. Ann N Y Acad Sci 539:468–470
MacDonald AB, Miranda JM (1987) Concurrent neocortical borreliosis and Alzheimer’s disease. Hum Pathol 18:759–761
Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet (London, England) 379:461–473
Radolf JD, Goldberg MS, Bourell K, Baker SI, Jones JD, Norgard MV (1995) Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun 63:2154–2163
Ramesh G, Alvarez AL, Roberts ED, Dennis VA, Lasater BL, Alvarez X, Philipp MT (2003) Pathogenesis of Lyme neuroborreliosis: Borrelia burgdorferi lipoproteins induce both proliferation and apoptosis in rhesus monkey astrocytes. Eur J Immunol 33:2539–2550
MacDonald AB (2007) Alzheimer’s neuroborreliosis with trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med Hypotheses 68:822–825
Appelt DM, Roupas MR, Way DS, Bell MG, Albert EV, Hammond CJ, Balin BJ (2008) Inhibition of apoptosis in neuronal cells infected with Chlamydophila (Chlamydia) pneumoniae. BMC Neurosci 9:13
Paradowski B, Jaremko M, Dobosz T, Leszek J, Noga L (2007) Evaluation of CSF-Chlamydia pneumoniae, CSF-tau, and CSF-Abeta42 in Alzheimer’s disease and vascular dementia. J Neurol 254:154–159
Shima K, Kuhlenbäumer G, Rupp J (2010) Chlamydia pneumoniae infection and Alzheimer’s disease: a connection to remember? Med Microbiol Immunol 199:283–289
Hammond CJ, Hallock LR, Howanski RJ, Appelt DM, Little CS, Balin BJ (2010) Immunohistological detection of Chlamydia pneumoniae in the Alzheimer’s disease brain. BMC Neurosci 11:121
Pollack DV, Croteau NL, Stuart ES (2008) Uptake and intra-inclusion accumulation of exogenous immunoglobulin by Chlamydia-infected cells. BMC Microbiol 8:213
Balin BJ, Gérard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, Whittum-Hudson JA, Hudson AP (1998) Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol 187:23–42
Albert NM (2000) Inflammation and infection in acute coronary syndrome. J Cardiovasc Nurs 15:13–26
MacIntyre A, Abramov R, Hammond CJ, Hudson AP, Arking EJ, Little CS, Appelt DM, Balin BJ (2003) Chlamydia pneumoniae infection promotes the transmigration of monocytes through human brain endothelial cells. J Neurosci Res 71:740–750
Manabe T, Mizukami K, Akatsu H, Teramoto S, Yamaoka K, Nakamura S, Ohkubo T, Kudo K et al (2016) Influence of pneumonia complications on the prognosis of patients with autopsy-confirmed Alzheimer’s disease, dementia with Lewy bodies, and vascular dementia. Psychogeriatrics 16:305–314
Dreses-Werringloer U, Bhuiyan M, Zhao Y, Gérard HC, Whittum-Hudson JA, Hudson AP (2009) Initial characterization of Chlamydophila (Chlamydia) pneumoniae cultured from the late-onset Alzheimer brain. Int J Med Microbiol: IJMM 299:187–201
Gérard HC, Dreses-Werringloer U, Wildt KS, Deka S, Oszust C, Balin BJ, Frey WH, Bordayo EZ et al (2006) Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol Med Microbiol 48:355–366
Takeda S, Sato N, Morishita R (2014) Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 6(171)
Alzforum | Networking for a cure. http://www.alzforum.org/papers/uber-eine-eigenartige-erkrankung-der-hirnrinde.
Licastro F, Porcellini E (2016) Persistent infections, immune-senescence and Alzheimer’s disease. Oncoscience 3:135–142
Fischer O (1907) Miliare nekrosen mit drusigen wucherungen der neurofibrillen, eine regelmässige veränderung der hirnrinde bei seniler demenz. Monatsschr Psychiatr Neurol 22:361–372
Fischer O (1910) Die presbyophrene demenz, deren anatomische grundlage und klinische abgrenzung. Z Gesamte Neurol Psychiatr 3:371–471
Goeman J, Hoksbergen I, Pickut BA, Dom L, Crols R, De Deyn PP (1996) Dementia paralytica in a fifteen-year-old boy. J Neurol Sci 144:214–217
Noguchi H, Moore JW (1913) A demonstration of Treponema pallidum in the brain in cases of general paralysis. J Exp Med 17:232–238
Möhle L, Israel N, Paarmann K, Krohn M, Pietkiewicz S, Müller A, Lavrik IN, Buguliskis JS et al (2016) Chronic Toxoplasma gondii infection enhances β-amyloid phagocytosis and clearance by recruited monocytes. Acta Neuropathol Commun 4(25):25
Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L (2015) Different brain regions are infected with fungi in Alzheimer’s disease. Sci Rep 5(15015)
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet (London, England) 1:1311–1315
Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D et al (2009) Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci 119:765–777
Franceschi F, Gasbarrini A, Polyzos SA, Kountouras J (2015) Extragastric diseases and Helicobacter pylori. Helicobacter 20(Suppl 1):40–46
Polepalle T, Moogala S, Boggarapu S, Pesala DS, Palagi FB (2015) Acute phase proteins and their role in periodontitis: a review. J Clin Diagn Res 9:ZE01–ZE05
Fenesy KE (1998) Periodontal disease: an overview for physicians. Mount Sinai J Med N Y 65:362–369
Kornhuber HH (1996) Propionibacterium acnes in the cortex of patients with Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 246:108–109
Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ (2008) Bacterial profiles of root caries in elderly patients. J Clin Microbiol 46:2015–2021
Delahaye F, Fol S, Célard M, Vandenesch F, Beaune J, Bozio A, de Gevigney G (2005) Propionibacterium acnes infective endocarditis. Study of 11 cases and review of literature. Arch Mal Coeur Vaiss 98:1212–1218
Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, Inflammation d LMJ (2008) Alzheimer’s disease: possible role of periodontal diseases. Alzheimer’s Dement 4:242–250
Gurav AN (2014) Alzheimer’s disease and periodontitis--an elusive link. Revista Da Associação Médica Brasileira (1992) 60:173–180
Hatipoglu MG, Kabay SC, Güven G (2011) The clinical evaluation of the oral status in Alzheimer-type dementia patients. Gerodontology 28:302–306
Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ (2008) Alzheimer’s disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimer’s Dis: JAD 13:437–449
Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P et al (2016) Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One 11:e0151081
Wu Z, Nakanishi H (2014) Connection between periodontitis and Alzheimer’s disease: possible roles of microglia and leptomeningeal cells. J Pharmacol Sci 126:8–13
Farhad SZ, Amini S, Khalilian A, Barekatain M, Mafi M, Barekatain M, Rafei E (2014) The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dental Res J 11:549–552
Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L et al (2009) TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol 216:92–97
Alonso R, Pisa D, Marina AI, Morato E, Rábano A, Carrasco L (2014) Fungal infection in patients with Alzheimer’s disease. J Alzheimer’s Dis: JAD 41:301–311
Panackal AA, Williamson PR (2015) Fungal infections of the central nervous system. Continuum (Minneapolis, Minn) 21:1662–1678
Prandota J (2010) Autism spectrum disorders may be due to cerebral toxoplasmosis associated with chronic neuroinflammation causing persistent hypercytokinemia that resulted in an increased lipid peroxidation, oxidative stress, and depressed metabolism of endogenous and exogenous substances. Res Autism Spectr Disord 4:119–155
Prandota J (2010) Neuropathological changes and clinical features of autism spectrum disorder participants are similar to that reported in congenital and chronic cerebral toxoplasmosis in humans and mice. Res Autism Spectr Disord 4:103–118
Prandota J (2011) Metabolic, immune, epigenetic, endocrine and phenotypic abnormalities found in individuals with autism spectrum disorders, Down syndrome and Alzheimer disease may be caused by congenital and/or acquired chronic cerebral toxoplasmosis. Res Autism Spectr Disord 5:14–59
Prandota J (2014) Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am J Alzheimer’s Dis Other Dementias 29:205–214
Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973
Sequiera LW, Carrasco LH, Curry A, Jennings LC, Lord MA, Sutton RNP (1979) Detection of herpes-simplex viral genome in brain tissue. Lancet 314:609–612
Ball MJ (1982) Limbic predilection in Alzheimer dementia: Is reactivated herpesvirus involved? Can J Neurol Sci 9:303–306
Gannicliffe A, Sutton RN, Itzhaki RF (1986) Viruses, brain and immunosuppression. Psychol Med 16:247–249
Agostini S, Mancuso R, Baglio F, Clerici M (2017) A protective role for herpes simplex virus type-1-specific humoral immunity in Alzheimer’s disease. Expert Rev Anti-Infect Ther 15:89–91
Itzhaki RF (2014) Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Front Aging Neurosci 6(202)
Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA (1997) Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet (London, England) 349:241–244
Koronyo Y, Salumbides BC, Black KL, Koronyo-Hamaoui M (2012) Alzheimer’s disease in the retina: imaging retinal Aß plaques for early diagnosis and therapy assessment. Neurodegener Dis 10:285–293
Olsson J, Lövheim H, Honkala E, Karhunen PJ, Elgh F, Kok EH (2016) HSV presence in brains of individuals without dementia: the TASTY brain series. Dis Model Mech 9:1349–1355
Itzhaki RF, Wozniak MA (2012) Could antivirals be used to treat Alzheimer’s disease? Future Microbiol 7:307–309
Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB (2007) Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett 429:95–100
Wozniak MA, Mee AP, Itzhaki RF (2009) Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol 217:131–138
Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF (2005) Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J Med Virol 75:300–306
Lövheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F (2015) Herpes simplex infection and the risk of Alzheimer’s disease: a nested case-control study. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11:587–592
Lövheim H, Gilthorpe J, Adolfsson R, Nilsson L-G, Elgh F (2015) Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimer’s Dementia 11:593–599
Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, Tyler KL (1998) Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 51:554–559
Rodríguez-Violante M, Ordoñez G, Bermudez JR, Sotelo J, Corona T (2009) Association of a history of varicella virus infection with multiple sclerosis. Clin Neurol Neurosurg 111:54–56
Kristen H, Santana S, Sastre I, Recuero M, Bullido MJ, Aldudo J (2015) Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol Aging 36:2737–2747
Nimgaonkar VL, Yolken RH, Wang T, Chung-Chou HC, McClain L, McDade E, Snitz BE, Ganguli M Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Dis Assoc Disord 2015
Biesiada G, Czepiel J, Sobczyk-Krupiarz I, Mach T, Garlicki A. [Neurological complications among patients with zoster hospitalized in Department of Infectious Diseases in Cracow in 2001–2006]. Przegla̧d Lekarski 67: 149–150, 2010.
Barnes LL, Capuano AW, Aiello AE, Turner AD, Yolken RH, Torrey EF, Bennett DA (2015) Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis 211:230–237
Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, Schneider JA (2013) Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 208:564–572
Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, Licastro F (2014) Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging 35:122–129
Chiu WC, Tsan YT, Tsai SL, Chang CJ, Wang JD, Chen PC, Health Data Analysis in Taiwan Research G (2014) Hepatitis C viral infection and the risk of dementia. Eur J Neurol 21:1068–1e59
Senzolo M, Schiff S, D'Aloiso CM, Crivellin C, Cholongitas E, Burra P, Montagnese S (2011) Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol 17:3369–3374
Fletcher NF, McKeating JA (2012) Hepatitis C virus and the brain. J Viral Hepat 19:301–306
Grover VPB, Pavese N, Koh SB, Wylezinska M, Saxby BK, Gerhard A, Forton DM, Brooks DJ et al (2012) Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J Viral Hepat 19:e89–e96
Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, Watterson S et al (2013) The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38:106–118
Liu S-Y, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H et al (2013) Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38:92–105
Papassotiropoulos A, Lambert J-C, Wavrant-De Vrièze F, Wollmer MA, von der Kammer H, Streffer JR, Maddalena A, Huynh K-D, Wolleb S, Lutjohann D, Schneider B, Thal DR, Grimaldi LME, Tsolaki M, Kapaki E, Ravid R, Konietzko U, Hegi T, Pasch T, Jung H, Braak H, Amouyel P, Rogaev EI, Hardy J, Hock C, Nitsch RM. Cholesterol 25-hydroxylase on chromosome 10q is a susceptibility gene for sporadic Alzheimer’s disease. Neurodegener Dis 2: 233–241, 2005.
Lathe R, Sapronova A, Kotelevtsev Y (2014) Atherosclerosis and Alzheimer--diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr 14(36)
Friedman JE, Zabriskie JB, Plank C, Ablashi D, Whitman J, Shahan B, Edgell R, Shieh M et al (2005) A randomized clinical trial of valacyclovir in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 11:286–295
Wozniak MA, Itzhaki RF (2010) Antiviral agents in Alzheimer’s disease: hope for the future? Ther Adv Neurol Disord 3:141–152
Devi G, Schultz S, Khosrowshahi L, Agnew A, Olali E (2008) A retrospective chart review of the tolerability and efficacy of intravenous immunoglobulin in the treatment of Alzheimer’s disease. J Am Geriatr Soc 56:772–774
Fillit H, Hess G, Hill J, Bonnet P, Toso C (2009) IV immunoglobulin is associated with a reduced risk of Alzheimer disease and related disorders. Neurology 73:180–185
Leszek J, Inglot AD, Janusz M, Byczkiewicz F, Kiejna A, Georgiades J, Lisowski J. Colostrinin proline-rich polypeptide complex from ovine colostrum--a long-term study of its efficacy in Alzheimer’s disease. Med Sci Monit 8: PI93–96, 2002.
Leszek J, Inglot AD, Janusz M, Lisowski J, Krukowska K, Georgiades JA (1999) Colostrinin: a proline-rich polypeptide (PRP) complex isolated from ovine colostrum for treatment of Alzheimer’s disease. A double-blind, placebo-controlled study. Arch Immunol Ther Exp 47:377–385
Sochocka M, Zaczyńska E, Leszek J, Siemieniec I, Błach-Olszewska Z (2008) Effect of donepezil on innate antiviral immunity of human leukocytes. J Neurol Sci 273:75–80
Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D (2005) Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp Gerontol 40:165–171
Jiang L, Miao Z, Kimura RH, Liu H, Cochran JR, Culter CS, Bao A, Li P et al (2011) Preliminary evaluation of (177)Lu-labeled knottin peptides for integrin receptor-targeted radionuclide therapy. Eur J Nucl Med Mol Imaging 38:613–622
Jiang Y, Zou Y, Chen S, Zhu C, Wu A, Liu Y, Ma L, Zhu D et al (2013) The anti-inflammatory effect of donepezil on experimental autoimmune encephalomyelitis in C57 BL/6 mice. Neuropharmacology 73:415–424
Yoshiyama Y, Kojima A, Ishikawa C, Arai K (2010) Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J Alzheimer's Dis: JAD 22:295–306
Saxena G, Singh SP, Agrawal R, Nath C (2008) Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol 581:283–289
Meunier J, Ieni J, Maurice T (2006) The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br J Pharmacol 149:998–1012
Hwang J, Hwang H, Lee H-W, Suk K (2010) Microglia signaling as a target of donepezil. Neuropharmacology 58:1122–1129
Butt AM, Fern RF, Matute C (2014) Neurotransmitter signaling in white matter. Glia 62:1762–1779
Hösli L, Hösli E, Käser H (1993) Colocalization of cholinergic, adrenergic and peptidergic receptors on astrocytes. Neuroreport 4:679–682
Kim HG, Moon M, Choi JG, Park G, Kim A-J, Hur J, Lee K-T, Oh MS (2014) Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology 40:23–32
Carnevale D, De Simone R, Minghetti L (2007) Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Dis Drug Targets 6:388–397
Haddad JJ, Saadé NE, Safieh-Garabedian B (2002) Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol 133:1–19
Ashraf GM, Perveen A, Zaidi SK, Tabrez S, Kamal MA, Banu N (2015) Studies on the role of goat heart galectin-1 as an erythrocyte membrane perturbing agent. Saudi J Biol Sci 22:112–116
Szekely CA, Zandi PP (2010) Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol Disord Drug Targets 9:132–139
Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA, Group MS (2005) Nonsteroidal anti-inflammatory drug use and Alzheimer’s disease risk: the MIRAGE Study. BMC Geriatr 5(2)
Newman DJ, Cragg GM (2004) Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr Med Chem 11:1693–1713
Abraham J, Johnson RW (2009) Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res 12:445–453
Korada SK, Yarla NS, Bishayee A, Aliev G, Aruna Lakshmi K, Arunasree MK, Dananajaya BL, Mishra V (2016) Can probiotics cure inflammatory bowel diseases? Curr Pharm Des 22:904–917
Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, Djalali M et al (2015) Effects of probiotics on biomarkers of oxidative stress and inflammatory factors in petrochemical workers: a randomized, double-blind, placebo-controlled trial. Int J Prev Med 6:82
Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I (2013) Antipathogenic activity of probiotics against Salmonella Typhimurium and Clostridium difficile in anaerobic batch culture systems: is it due to synergies in probiotic mixtures or the specificity of single strains? Anaerobe 24:60–65
Mallikarjuna N, Praveen K, Yellamma K (2016) Role of Lactobacillus plantarum MTCC1325 in membrane-bound transport ATPases system in Alzheimer’s disease-induced rat brain. BioImpacts: BI 6:203–209
Acknowledgments
The authors are very grateful for the animal facilities that were provided by the center for preclinical trials of IPAC RAS.
Funding
This work was supported by the Russian Academic Excellence project “5-100” for the Sechenov University, Moscow, Russian Federation. This research was also supported in part by the RSF project #14-23-00160P and the scientific projects of IPAC (topics 48.8. and 48.9). Part of this work was also supported by the project of RAS Program Fundamental Research for Biomedical Technologies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ashraf, G.M., Tarasov, V.V., Makhmutovа, A. et al. The Possibility of an Infectious Etiology of Alzheimer Disease. Mol Neurobiol 56, 4479–4491 (2019). https://doi.org/10.1007/s12035-018-1388-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1388-y