Abstract
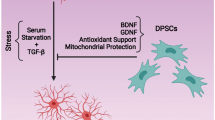
Dental pulp stem cells (DPSCs) are promising for use in neurodegenerative-diseases because of their neural crest origin. While neuronal differentiation of DPSCs has been shown, their plasticity towards astrocyte-like cells remains to be studied. We aimed to examine differentiation potential of DPSCs to astrocytes and their consequent neuroprotective role towards dopaminergic (DA) neurons under 6-hydroxydopamine (6-OHDA) toxicity. Induction of DPSCs to astrocytes with differentiation factors showed definitive increase in astrocyte-specific markers glial fibrillary acidic protein (GFAP), and excitatory amino acid transporter 2 along with glial calcium-binding protein S100β through FACS and immunofluorescence assays. RT-PCR and ELISA showed significant increase in BDNF and GDNF expression and secretion in astrocyte-differentiated DPSCs over naïve DPSCs. Neuroprotective role of these cells on DA neurons under 6-OHDA stress was evaluated by both contact and non-contact methods. FACS analysis of PKH26-stained SH-SY5Y homogenous cells in contact method and of TH immunopositive cells in primary midbrain culture in non-contact method both indicated higher survival of DA neurons in astrocyte-differentiated DPSCs over naïve DPSCs. Recovery of β-tubulin III and TH immunopositive cells was reduced in the presence of TrkB inhibitor, suggesting a key neuroprotective role of BDNF secretion by DPSCs. When nitric oxide (NO) release was inhibited by l-NAME in primary midbrain culture, BDNF release in co-culture under 6-OHDA stress reduced further in naïve DPSCs than in astrocyte-differentiated DPSCs, suggesting that BDNF release in naïve DPSCs is primarily regulated by paracrine signaling while for differentiated DPSCs, it is equally through autocrine and paracrine signaling with NO being the mediator. In conclusion, we suggest that DPSCs exposed to glial commitment cues exhibit substantial differentiation towards astrocyte-like cells with better neuroprotective activity against 6-OHDA toxicity than naïve DPSCs.











Similar content being viewed by others
References
Barreto GE, Gonzalez J, Torres Y, Morales L (2011) Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res 71(2):107–113
Hamby ME, Sofroniew MV (2010) Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 7(4):494–506
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8):626–640
Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M (2008) Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci 105(9):3581–3586
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20(12):570–577
Rappold PM, Tieu K (2010) Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 7(4):413–423
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10(5):615–622
Pehar M, Beeson G, Beeson CC, Johnson JA, Vargas MR (2014) Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G93AAstrocytes without extending the survival of ALS-linked mutant hSOD1 mice. PLoS One 9(7):e103438
Booth HDE, Hirst WD, Wade-Martins R (2017) The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci 40(6):358–370
Choi I, Choi DJ, Yang H, Woo JH, Chang MY, Kim JY, Sun W, Park SM et al (2016) PINK1 expression increases during brain development and stem cell differentiation, and affects the development of GFAP-positive astrocytes. Mol Brain 9:5
Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E, Hirst WD (2015) Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet 24(21):6013–6028
Kim KS, Kim JS, Park JY, Suh YH, Jou I, Joe EH, Park SM (2013) DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet 22(23):4805–4817
Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH (2002) Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J Neurochem 83(6):1431–1440
Lev N, Barhum Y, Ben-Zur T, Melamed E, Steiner I, Offen D (2013) Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci 50(3):542–550
Miklossy J, Arai T, Guo JP, Klegeris A, Yu S, McGeer EG, McGeer PL (2006) LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol 65(10):953–963
Yun HM, Choi DY, Oh KW, Hong JT (2015) PRDX6 exacerbates dopaminergic neurodegeneration in a MPTP mouse model of Parkinson’s disease. Mol Neurobiol 52(1):422–431
Janebodin K, Horst OV, Ieronimakis N, Balasundaram G, Reesukumal K, Pratumvinit B, Reyes M (2011) Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One 6(11):e27526
Janebodin K, Reyes M (2012) Neural crest-derived dental pulp stem cells function as ectomesenchyme to support salivary gland tissue formation. Dentistry S13:001. https://doi.org/10.4172/2161-1122S13-001
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP et al (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127:1671–1679
Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L et al (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513(7519):551–554
Thesleff I, Tummers M. (2009) Tooth organogenesis and regeneration. In: StemBook from: https://www.ncbi.nlm.nih.gov/books/NBK27071/. https://doi.org/10.3824/stembook.1.37.1
Majumdar D, Kanafi M, Bhonde R, Gupta P, Datta I (2016) Differential neuronal plasticity of dental pulp stem cells from exfoliated deciduous and permanent teeth towards dopaminergic neurons. J Cell Physiol 231:2048–2063
Jung J, Kim JW, Moon HJ, Hong JY, Hyun JK (2016) Characterization of neurogenic potential of dental pulp stem cells cultured in xeno/serum-free condition: in vitro and in vivo assessment. Stem Cells Int 2016:6921097
Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA et al (2012) Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med 1(3):177–187
Young FI, Telezhkin V, Youde SJ, Langley MS, Stack M, Kemp PJ, Song B (2016) Clonal heterogeneity in the neuronal and glial differentiation of dental pulp stem/progenitor cells. Stem Cells Int 1290561
Kanafi M, Majumdar D, Bhonde R, Gupta P, Datta I (2014) Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. J Cell Physiol 229(10):1369–1377
Datta I, Mishra S, Mohanty L, Pulikkot S, Joshi PG (2011) Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy 13(8):918–932
Bahat-Stroomza M, Barhum Y, Levy YS, Karpov O, Bulvik S, Melamed E, Offen D (2009) Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson's disease. J Mol Neurosci 39(1–2):199–210
Datta I, Ganapathy K, Razdan R, Bhonde R (2018) Location and number of astrocytes determine dopaminergic neuron survival and function under 6-OHDA stress mediated through differential BDNF release. Mol Neurobiol 55(7):5505–5525
Ganapathy K, Datta I, Sowmithra S, Joshi P, Bhonde R (2016a) Influence of 6-hydroxydopamine toxicity on α-synuclein phosphorylation, resting vesicle expression, and vesicular dopamine release. J Cell Biochem 9999:1–18
Ganapathy K, Sowmithra S, Bhonde R, Datta I (2016b) By changing dimensionality, sequential culturing of midbrain cells, rather than two-dimensional culture, generates a neuron-glia ratio closer to in vivo adult midbrain. Cells Tissues Organs 201:445–463
Prasanna SJ, Saha B, Nandi D (2007) Involvement of oxidative and nitrosative stress in modulation of gene expression and functional responses by IFNγ. Int Immunol 19(7):867–879
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt. 5):2283–2301
German DC, Manaye KF, Sonsalla PK, Brooks BA (1992) Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci 648:42–62
Hirsch EC, Graybiel AM, Agid Y (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334:345–348
Hirsch EC, Graybiel AM, Agid Y (1989) Selective vulnerability of pigmented dopaminergic neurons in Parkinson’s disease. Acta Neurol Scand Suppl 126:19–22
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136:2419–2431
Damier P, Hirsch EC, Javoy-Agid F, Zhang P, Agid Y (1993) Protective role of glutathione peroxidase against neuronal death in Parkinson’s disease. Neuroscience 52:1–6
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26:1787–1795
Karaöz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 136:455–473
Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I et al (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 55:323–332
Farkas LM, Krieglstein K (2002) Heparin-binding epidermal growth factor-like growth factor (HB-EGF) regulates survival of midbrain dopaminergic neurons. J Neural Transm (Vienna) 109(3):267–277
Galvez-Contreras AY, Quiñones-Hinojosa A, Gonzalez-Perez O (2013) The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front Cell Neurosci 7:258
Kornblum HI, Zurcher SD, Werb Z, Derynck R, Seroogy KB (1999) Multiple trophic actions of heparin-binding epidermal growth factor (HB-EGF) in the central nervous system. Eur J Neurosci 11:3236–3246
Kornblum HI, Hussain R, Wiesen J, Miettinen P, Zurcher SD, Chow K, Derynck R, Werb Z (1998) Abnormal astrocyte development and neuronal death in mice lacking the epidermal growth factor receptor. J Neurosci Res 53(6):697–717
Angenieux B, Schorderet DF, Arsenijevic Y (2006) Epidermal growth factor is a neuronal differentiation factor for retinal stem cells in vitro. Stem Cells 24(3):696–706
Burrows RC, Wancio D, Levitt P, Lillien L (1997) Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron 19(2):251–267
Kilpatrick TJ, Bartlett PF (1995) Cloned multipotential precursors from the mouse cerebrum require FGF-2, whereas glial restricted precursors are stimulated with either FGF-2 or EGF. J Neurosci 15(5):3653–3661
Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH (1997) Epidermal growth factor and fibroblast growth Factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci 17(15):5820–5829
Shen YM, Meltzer H, Saljooque F, Sang U (2001) Stimulation of the epidermal growth factor receptor induces glial-specific protein expression in the human DAOY neuroectodermal cell line. Dev Neurosci 23(1):84–90
Agarwal A, Zhang M, Trembak-Duff I, Unterbarnscheidt T, Radyushkin K, Dibaj P, de Souza DM, Boretius S et al (2014) Dysregulated expression of neuregulin-1 by cortical pyramidal neurons disrupts synaptic plasticity. Cell Rep 8(4):1130–1145
Britsch S (2007) The neuregulin-I/ErbB signaling system in development and disease. Springer Science & Business Media 190:1–65
Mei L, Nave KA (2014) Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 83(1):27–49
Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC et al (2011) Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci 31(1):15–25
Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR (1997) Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology 138(1):19–25
Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ (2011) How factors secreted from astrocytes impact myelin repair. J Neurosci Res 89(1):13–21
Ojeda SR, Ma YJ (1999) Glial-neuronal interactions in the neuroendocrine control of mammalian puberty: facilitatory effects of gonadal steroids. J Neurobiol 40:528–540
Parker MW, Chen Y, Hallenbeck JM, Ford BD (2002) Neuregulin expression after focal stroke in the rat. Neurosci Lett 334(3):169–172
Schmid RS, McGrath B, Berechid BE, Boyles B, Marchionni M, Šestan N, Anton ES (2003) Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci 100(7):4251–4256
Thompson RJ, Roberts B, Alexander CL, Williams SK, Barnett SC (2000) Comparison of neuregulin-1 expression in olfactory ensheathing cells, Schwann cells and astrocytes. J Neurosci Res 61(2):172–185
Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, Oohira A (2001) Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. J Neurosci 21(4):1257–1264
Paco S, Hummel M, Plá V, Sumoy L, Aguado F (2016) Cyclic AMP signaling restricts activation and promotes maturation and antioxidant defenses in astrocytes. BMC Genomics 17:304
Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB (1998) Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol 53:355–369
Yoshida H, Imaizumi T, Tanji K, Sakaki H, Metoki N, Hatakeyama M, Yamashita K, Ishikawa A et al (2005) Platelet-activating factor enhances the expression of nerve growth factor in normal human astrocytes under hypoxia. Mol Brain Res 133(1):95–101
Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L (2007) Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 25(12):1468–1475
Rao M, Malik N (2013) Neural differentiation from pluripotent stem cells. InStem Cells Handbook. Springer, New York, pp. 149–160
Schwartz PH, Brick DJ, Stover AE, Loring JF, Müller FJ (2008) Differentiation of neural lineage cells from human pluripotent stem cells. Methods 45(2):142–158
Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD et al (2000) Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-κB. Mol Pharmacol 57(4):667–678
DeSilva TM, Borenstein NS, Volpe JJ, Kinney HC, Rosenberg PA (2012) Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development. J Comp Neurol 520(17):3912–3932
Jungblut M, Tiveron MC, Barral S, Abrahamsen B, Knöbel S, Pennartz S, Schmitz J, Perraut M et al (2012) Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia 60(6):894–907
Markiewicz I, Lukomska B (2006) The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp (Wars) 66(4):343–358
Wang DD, Bordey A (2008) The astrocyte odyssey. Prog Neurobiol 86(4):342–367
Ayers-Ringler JR, Jia YF, Qiu YY, Choi DS (2016) Role of astrocytic glutamate transporter in alcohol use disorder. World J Psychiatry 6(1):31–42
Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, Eljaafari A (2004) Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology 41:469–476
Fibbe WE, Noort WA (2003) Mesenchymal stem cells and hematopoietic stem cells transplantation. Ann N Y Acad Sci 996:235–244
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2(8):675–680
Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S et al (2002) Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat Immunol 3(7):673–680
Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA (2009) Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells 27(9):2229–2237
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA (2014) Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 9(10):e109305
Young F, Sloan A, Song B (2013) Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. J Neurosci Res 91(11):1383–1393
Kimelberg HK, Nedergaard M (2010) Functions of astrocytes and their potential as thera- peutic targets. Neurotherapeutics 7(4):338–353
Parpura V, Grubisic V, Verkhratsky A (2011) Ca(2+) sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta 1813(5):984–991
Ramaswamy S, Kordower JH (2009) Are growth factors the answer? Parkinsonism Relat Disord 15(Suppl 3):S176–S180
Yasuda T, Mochizuki H (2010) Use of growth factors for the treatment of Parkinson’s disease. Expert Rev Neurother 10(6):915–924
Yoshida K, Toya S (1997) Neurotrophic activity in cytokine-activated astrocytes. Keio J Med 46(2):55–60
Hausmann R, Riess R, Fieguth A, Betz P (2000) Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int J Legal Med 113(2):70–75
Wachter B, Schürger S, Rolinger J, von Ameln-Mayerhofer A, Berg D, Wagner HJ, Kueppers E (2010) Effect of 6-hydroxydopamine (6-OHDA) on proliferation of glial cells in the rat cortex and striatum: evidence for de-differentiation of resident astrocytes. Cell Tissue Res 342(2):147–160
Baquet ZC, Bickford PC, Jones KR (2005) Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci 25(26):6251–6259
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8(254):1–10
Feng L, Wang CY, Jiang H, Oho C, Mizuno K, Dugich-Djordjevic M, Lu B (1999) Differential effects of GDNF and BDNF on cultured ventral mesencephalic neurons. Brain Res Mol Brain Res 66:62–70
Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–232
Murer MG, Yan Q, Raisman-Vozari R (2001) Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol 63:71–124
Bal-Price A, Moneer Z, Brown GC (2002) Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia 40:312–323
Buskila Y, Amitai Y (2010) Astrocytic iNOS-dependent enhancement of synaptic release in mouse neocortex. J Neurophysiol 103:1322–1328
Ikeda H, Murase K (2004) Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J Neurosci 24:9888–9896
Mehta B, Begum G, Joshi NB, Joshi PG (2008) Nitric oxide–mediated modulation of synaptic activity by astrocytic P2Y receptors. J Gen Physiol 132(3):339–349
Xie AX, Petravicz J, McCarthy KD (2015) Molecular approaches for manipulating astrocytic signaling in vivo. Front Cell Neurosci 9:144
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Supplementary figure 1
FACS histograms for the astrocyte specific markers GFAP & EAAT2 along with glial calcium-binding protein S100β; neural commitment marker ASCL1; and neural crest marker HNK1 in DPSCs and astrocytes differentiated DPSCs at different day points of induction. (PNG 1166 kb)
Supplementary figure 2
FACS histograms of each cell population β tubulin III (total neuronal cells), GFAP (astrocytes) and TH (dopaminergic neurons) in the primary midbrain cells co-cultured with DPSC and astrocyte-differentiated DPSCs under 6-OHDA in comparison with primary midbrain cells in control and treated conditions. (PNG 1343 kb)
Supplementary figure 3
FACS histograms of each cell population with the TrkB inhibitor ANA12 A) β tubulin III (total neuronal cells) B) GFAP (astrocytes) and c) TH, (dopaminergic neurons) in the primary midbrain cells co-cultured with DPSC and astrocyte induced DPSCs under 6-OHDA. (PNG 673 kb)
Rights and permissions
About this article
Cite this article
Ganapathy, K., Datta, I. & Bhonde, R. Astrocyte-Like Cells Differentiated from Dental Pulp Stem Cells Protect Dopaminergic Neurons Against 6-Hydroxydopamine Toxicity. Mol Neurobiol 56, 4395–4413 (2019). https://doi.org/10.1007/s12035-018-1367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1367-3