Abstract
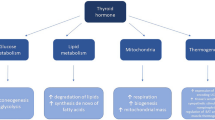
Thyroid hormones (THs) play a critical function in fundamental signaling of the body regulating process such as metabolism of glucose and lipids, cell maturation and proliferation, and neurogenesis, to name just a few. THs trigger biological effects both by directly affecting gene expression through the interaction with nuclear receptors (genomic effects) and by activating protein kinases and/or ion channels (short-term effects). For years, a close relationship between the THs hormones and the central nervous system (CNS) has been described, not only for neuronal cells but also for glial development and differentiation. A deficit in thyroid hormones triiodothyronine (T3) and thyroxine (T4) is observed in the hypothyroid condition, generated by a iodine deficiency or an autoimmune response of the body. In the hypothyroid condition, several cellular deregulation and alterations have been described in dendrite spine morphology, cell migration and proliferation, and impaired synaptic transmission in the hippocampus, among others. The aim of this review is to describe the role of the thyroid hormones with focus in brain function and neurodegenerative disorders.




Similar content being viewed by others
References
Bernal J (2000) Thyroid hormones in brain development and function. Endotext 3:1–48. https://doi.org/10.1038/ncpendmet0424
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170
Angelousi A, Kassi E, Nasiri-Ansari N, Weickert MO, Randeva H, Kaltsas G (2018) Clock genes alterations and endocrine disorders. Eur J Clin Invest 48:e12927. https://doi.org/10.1111/eci.12927
Lazarus JH (2002) Epidemiology and prevention of thyroid disease in pregnancy. Thyroid 12:861–865. https://doi.org/10.1089/105072502761016485
Vanderpump MPJ (2011) The epidemiology of thyroid disease. Br Med Bull 99:39–51. https://doi.org/10.1093/bmb/ldr030
Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, Steegers EAP, Visser TJ et al (2016) Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 4:35–43. https://doi.org/10.1016/S2213-8587(15)00327-7
Modesto T, Tiemeier H, Peeters RP, Jaddoe VWV, Hofman A, Verhulst FC, Ghassabian A (2015) Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 169:838–845. https://doi.org/10.1001/jamapediatrics.2015.0498
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30:376–408
Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S (2010) Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis 25:339–354
Yu J, Tang Y-Y, Feng H-B, Cheng X-X (2014) A behavioral and micro positron emission tomography imaging study in a rat model of hypothyroidism. Behav Brain Res 271:228–233. https://doi.org/10.1016/j.bbr.2014.06.019
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A et al (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22:421–426. https://doi.org/10.1038/nm.4057
Martínez-Sánchez N, Alvarez CV, Fernø J, Nogueiras R, Diéguez C, López M (2014) Hypothalamic effects of thyroid hormones on metabolism. Best Pract Res Clin Endocrinol Metab 28:703–712
Munhoz RP, Teive HAG, Troiano AR et al (2004) Parkinson’s disease and thyroid dysfunction. Parkinsonism Relat Disord 10:381–383. https://doi.org/10.1016/j.parkreldis.2004.03.008
Chaalal A, Poirier R, Blum D, Gillet B, le Blanc P, Basquin M, Buée L, Laroche S et al (2014) PTU-induced hypothyroidism in rats leads to several early neuropathological signs of Alzheimer’s disease in the hippocampus and spatial memory impairments. Hippocampus 24:1381–1393. https://doi.org/10.1002/hipo.22319
Garcia-Moreno JM, Chacon J (2002) Hypothyroidism concealed by Parkinson’s disease. Rev Neurol 35:741–742
Bernal J, Guadaño-Ferraz A, Morte B (2015) Thyroid hormone transporters-functions and clinical implications. Nat Rev Endocrinol 11:406–417
Hammes SR, Davis PJ (2015) Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract Res Clin Endocrinol Metab 29:581–593. https://doi.org/10.1016/j.beem.2015.04.001
Mullur R, Liu Y-Y, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382. https://doi.org/10.1152/physrev.00030.2013
Bowers J, Terrien J, Clerget-Froidevaux MS, Gothié JD, Rozing MP, Westendorp RGJ, van Heemst D, Demeneix BA (2013) Thyroid hormone signaling and homeostasis during aging. Endocr Rev 34:556–589
Jansen J, Friesema ECH, Milici C, Visser TJ (2005) Thyroid hormone transporters in health and disease. Thyroid 15:757–768. https://doi.org/10.1089/thy.2005.15.757
Bernal J (2002) Action of thyroid hormone in brain. J Endocrinol Investig 25:268–288. https://doi.org/10.1007/BF03344003
Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE (2014) Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol 10:582–591
Alvarez-Dolado M, Ruiz M, Del Río JA et al (1999) Thyroid hormone regulates reelin and dab1 expression during brain development. J Neurosci 19:6979–6993
Sui L, Ren W-W, Li B-M (2010) Administration of thyroid hormone increases reelin and brain-derived neurotrophic factor expression in rat hippocampus in vivo. Brain Res 1313:9–24. https://doi.org/10.1016/j.brainres.2009.12.010
Pathak A, Sinha RA, Mohan V, Mitra K, Godbole MM (2011) Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb Cortex 21:11–21. https://doi.org/10.1093/cercor/bhq052
Gil-Ibáñez P, Bernal J, Morte B (2014) Thyroid hormone regulation of gene expression in primary cerebrocortical cells: role of thyroid hormone receptor subtypes and interactions with retinoic acid and glucocorticoids. PLoS One 9. https://doi.org/10.1371/journal.pone.0091692
Diez D, Grijota-Martinez C, Agretti P, de Marco G, Tonacchera M, Pinchera A, Morreale de Escobar G, Bernal J et al (2008) Thyroid hormone action in the adult brain: gene expression profiling of the effects of single and multiple doses of triiodo-L-thyronine in the rat striatum. Endocrinology 149:3989–4000. https://doi.org/10.1210/en.2008-0350
Potter GB, Zarach JM, Sisk JM, Thompson CC (2002) The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol 16:2547–2560. https://doi.org/10.1210/me.2002-0115
Bonett RM, Hu F, Bagamasbad P, Denver RJ (2009) Stressor and glucocorticoid-dependent induction of the immediate early gene kruppel-like factor 9: implications for neural development and plasticity. Endocrinology 150:1757–1765. https://doi.org/10.1210/en.2008-1441
Gilbert ME, Sanchez-Huerta K, Wood C (2016) Mild thyroid hormone insufficiency during development compromises activity-dependent neuroplasticity in the hippocampus of adult male rats. Endocrinology 157:774–787. https://doi.org/10.1210/en.2015-1643
Desouza LA, Sathanoori M, Kapoor R, Rajadhyaksha N, Gonzalez LE, Kottmann AH, Tole S, Vaidya VA (2011) Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult mammalian brain. Endocrinology 152:1989–2000. https://doi.org/10.1210/en.2010-1396
Kaleka KS, Gerges NZ (2016) Neurogranin restores amyloid β-mediated synaptic transmission and long-term potentiation deficits. Exp Neurol 277:115–123. https://doi.org/10.1016/j.expneurol.2015.12.013
Farwell AP, Tranter MP, Leonard JL (1995) Thyroxine-dependent regulation of integrin-laminin interactions in astrocytes. Endocrinology 136:3909–3915. https://doi.org/10.1210/endo.136.9.7649099
Farwell AP, Dubord-Tomasetti SA (1999) Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology 140:4221–4227. https://doi.org/10.1210/endo.140.9.7007
Royland JE, Parker JS, Gilbert ME (2008) A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338. https://doi.org/10.1111/j.1365-2826.2008.01793.x
Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043. https://doi.org/10.1172/JCI60047
Dräger UC (2006) Retinoic acid signaling in the functioning brain. Sci STKE 2006(324):pe10
Fuhrer D, Brix K, Biebermann H (2015) Understanding the healthy thyroid state in 2015. Eur Thyroid J 4:1–8. https://doi.org/10.1159/000431318
Gereben B, McAninch EA, Ribeiro MO, Bianco AC (2015) Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol 11:642–652
Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM (2002) Psychological well-being in patients on “adequate” doses of L-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol 57:577–585. https://doi.org/10.1046/j.1365-2265.2002.01654.x
Blum MR, Wijsman LW, Virgini VS, Bauer DC, den Elzen WPJ, Jukema JW, Buckley BM, de Craen AJM et al (2016) Subclinical thyroid dysfunction and depressive symptoms among the elderly: a prospective cohort study. Neuroendocrinology 103:291–299. https://doi.org/10.1159/000437387
Jia Y, Zhong S, Wang Y, Liu T, Liao X, Huang L (2015) The correlation between biochemical abnormalities in frontal white matter, hippocampus and serum thyroid hormone levels in first-episode patients with major depressive disorder. J Affect Disord 180:162–169. https://doi.org/10.1016/j.jad.2015.04.005
Kratzsch J, Pulzer F (2008) Thyroid gland development and defects. Best Pract Res Clin Endocrinol Metab 22:57–75. https://doi.org/10.1016/j.beem.2007.08.006
Farahvar A, Meisami E (2007) Novel two-dimensional morphometric maps and quantitative analysis reveal marked growth and structural recovery of the rat hippocampal regions from early hypothyroid retardation. Exp Neurol 204:541–555. https://doi.org/10.1016/j.expneurol.2006.10.012
Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM (1991) Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol 314:171–186. https://doi.org/10.1002/cne.903140116
Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM (1992) Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322:501–518. https://doi.org/10.1002/cne.903220405
Rodger J, Salvatore L, Migani P (2012) Should i stay or should i go? Ephs and ephrins in neuronal migration. NeuroSignals 20:190–201. https://doi.org/10.1159/000333784
Shiraki A, Saito F, Akane H, Takeyoshi M, Imatanaka N, Itahashi M, Yoshida T, Shibutani M (2014) Expression alterations of genes on both neuronal and glial development in rats after developmental exposure to 6-propyl-2-thiouracil. Toxicol Lett 228:225–234. https://doi.org/10.1016/j.toxlet.2014.04.018
Chen C, Zhou Z, Zhong M, Zhang Y, Li M, Zhang L, Qu M, Yang J et al (2012) Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRα1. Stem Cells Dev 21:2667–2681. https://doi.org/10.1089/scd.2012.0023
Gilbert ME, Sui L (2006) Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 1069:10–22. https://doi.org/10.1016/j.brainres.2005.10.049
Gilbert ME (2004) Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Dev Brain Res 148:11–18. https://doi.org/10.1016/j.devbrainres.2003.09.018
Sui L, Anderson WL, Gilbert ME (2005) Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci 85:647–656. https://doi.org/10.1093/toxsci/kfi095
Amano I, Takatsuru Y, Khairinisa MA, et al (2018) Effects of mild perinatal hypothyroidism on cognitive function of adult male offspring. Endocrinology 159(4):1910–1921. https://doi.org/10.1210/en.2017-03125
Kawahori K, Hashimoto K, Yuan X et al (2018) Mild maternal hypothyroxinemia during pregnancy induces persistent DNA hypermethylation in the hippocampal brain-derived neurotrophic factor gene in mouse offspring. Thyroid 28:395–406. https://doi.org/10.1089/thy.2017.0331
Salazar P, Cisternas P, Codocedo JF, Inestrosa NC (2017) Induction of hypothyroidism during early postnatal stages triggers a decrease in cognitive performance by decreasing hippocampal synaptic plasticity. Biochim Biophys Acta Mol basis Dis 1863:870–883. https://doi.org/10.1016/j.bbadis.2017.01.002
Wang C (2013) The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013:390534
Iwen KA, Schröder E, Brabant G (2013) Thyroid hormones and the metabolic syndrome. Eur Thyroid J 2:83–92. https://doi.org/10.1159/000351249
Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR (1993) Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril 59:1187–1190. https://doi.org/10.1016/S0015-0282(16)55974-3
Mellert W, Deckardt K, Walter J, Gfatter S, van Ravenzwaay B (2003) Detection of endocrine-modulating effects of the antithyroid acting drug 6-propyl-2-thiouracil in rats, based on the “Enhanced OECD Test Guideline 407”. Regul Toxicol Pharmacol 38:368–377
Bauer M, Silverman DHS, Schlagenhauf F, London ED, Geist CL, van Herle K, Rasgon N, Martinez D et al (2009) Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab 94:2922–2929. https://doi.org/10.1210/jc.2008-2235
Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641
Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ et al (2002) Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 52:478–502
Miao Q, Zhang S, Guan YH, Ye HY, Zhang ZY, Zhang QY, Xue RD, Zeng MF et al (2011) Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. AJNR Am J Neuroradiol 32:1034–1042. https://doi.org/10.3174/ajnr.A2449
Khan JY, Rajakumar RA, Devaskar UP, Weissfeld LA, Devaskar SU (1999) Effect of primary congenital hypothyroidism upon expression of genes mediating murine brain glucose uptake. Pediatr Res 45:718–725
Mooradian AD, Girgis W, Shah GN (1997) Thyroid hormone-induced GLUT-1 expression in rat cerebral tissue: effect of age. Brain Res 747:144–146. https://doi.org/10.1016/S0006-8993(96)01110-9
Yau S, Li A, So K-F (2015) Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast 2015:717958–717913. https://doi.org/10.1155/2015/717958
Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA (2010) Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J 24:4793–4805. https://doi.org/10.1096/fj.10-161802
Munoz A, Wrighton C, Seliger B et al (1993) Thyroid hormone receptor/c-erbA: Control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J Cell Biol 121:423–438
Sjögren M, Alkemade A, Mittag J, Nordström K, Katz A, Rozell B, Westerblad H, Arner A et al (2007) Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J 26:4535–4545. https://doi.org/10.1038/sj.emboj.7601882
Varela L, Martínez-Sánchez N, Gallego R, Vázquez MJ, Roa J, Gándara M, Schoenmakers E, Nogueiras R et al (2012) Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J Pathol 227:209–222. https://doi.org/10.1002/path.3984
Biessels GJ, Kappelle LJ (2005) Increased risk of Alzheimer’s disease in type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans 33:1041–1044
Panveloski-Costa AC, Silva Teixeira S, Ribeiro IMR, Serrano-Nascimento C, das Neves RX, Favaro RR, Seelaender M, Antunes VR et al (2016) Thyroid hormone reduces inflammatory cytokines improving glycaemia control in alloxan-induced diabetic wistar rats. Acta Physiol 217:130–140. https://doi.org/10.1111/apha.12647
Prieto-Almeida F, Panveloski-Costa AC, Crunfli F, da Silva Teixeira S, Nunes MT, Torrão AS (2018) Thyroid hormone improves insulin signaling and reduces the activation of neurodegenerative pathway in the hippocampus of diabetic adult male rats. Life Sci 192:253–258. https://doi.org/10.1016/j.lfs.2017.11.013
Tan ZS, Beiser A, Vasan RS et al (2008) Thyroid function and the risk of Alzheimer disease: The Framingham study. Arch Intern Med 168:1514–1520. https://doi.org/10.1001/archinte.168.14.1514
Li X, Sundquist J, Sundquist K (2012) Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener Dis 10:277–284. https://doi.org/10.1159/000333222
Andersen SL, Laurberg P, Wu CS, Olsen J (2014) Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121:1365–1374. https://doi.org/10.1111/1471-0528.12681
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG (2014) The epidemiology and global burden of autism spectrum disorders. Psychol Med 45:1–13. https://doi.org/10.1017/s003329171400172x
Khan A, Harney JW, Zavacki AM, Sajdel-Sulkowska EM (2014) Disrupted brain thyroid hormone homeostasis and altered thyroid hormone-dependent brain gene expression in autism spectrum disorders. J Physiol Pharmacol 65:257–272
Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N (2006) Review of animal models for autism: implication of thyroid hormone. Congenit Anom (Kyoto) 46:1–9
Wu S, Ding Y, Wu F, Li R, Xie G, Hou J, Mao P (2015) Family history of autoimmune diseases is associated with an increased risk of autism in children: a systematic review and meta-analysis. Neurosci Biobehav Rev 55:322–332
Roman GC, Ghassabian A, Bongers-Schokking JJ et al (2013) Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 74:733–742. https://doi.org/10.1002/ana.23976
Brown AS, Surcel H-M, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Bao Y, Sourander A (2015) Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuro-Psychopharmacol Biol Psychiatry 57:86–92. https://doi.org/10.1016/j.pnpbp.2014.10.010
Molloy CA, Morrow AL, Meinzen-Derr J, Dawson G, Bernier R, Dunn M, Hyman SL, McMahon WM et al (2006) Familial autoimmune thyroid disease as a risk factor for regression in children with autism spectrum disorder: a CPEA study. J Autism Dev Disord 36:317–324. https://doi.org/10.1007/s10803-005-0071-0
Heinzel S, Roeben B, Ben-Shlomo Y, Lerche S, Alves G, Barone P, Behnke S, Berendse HW et al (2016) Prodromal markers in Parkinson’s disease: limitations in longitudinal studies and lessons learned. Front Aging Neurosci 8:147
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139(Suppl 1):318–324
Berger JR, Kelley RE (1981) Thyroid function in Parkinson disease. Neurology 31:93–95. https://doi.org/10.1212/WNL.31.1.93
Fernández E, García-Moreno J-M, Martín de Pablos A, Chacón J (2014) May the thyroid gland and thyroperoxidase participate in nitrosylation of serum proteins and sporadic Parkinson’s disease? Antioxid Redox Signal 21:2143–2148. https://doi.org/10.1089/ars.2014.6072
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344. https://doi.org/10.1056/NEJMra0909142
Cerpa W, Dinamarca MC, Inestrosa NC et al (2008) Structure-function implications in Alzheimer’s disease: effect of Abeta oligomers at central synapses. Curr Alzheimer Res 5:233–243. https://doi.org/10.2174/156720508784533321
Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 108:21–43. https://doi.org/10.1016/j.pneurobio.2013.06.004
Qiu C, Kivipelto M, Von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11:111–128
Contreras-Jurado C, Pascual A (2012) Thyroid hormone regulation of APP (β-amyloid precursor protein) gene expression in brain and brain cultured cells. Neurochem Int 60:484–487. https://doi.org/10.1016/j.neuint.2012.01.027
Ghenimi N, Alfos S, Redonnet A et al (2010) Adult-onset hypothyroidism induces the amyloidogenic pathway of amyloid precursor protein processing in the rat hippocampus. J Neuroendocrinol 22:951–959. https://doi.org/10.1111/j.1365-2826.2010.02002.x
Sampaolo S, Campos-Barros A, Mazziotti G, Carlomagno S, Sannino V, Amato G, Carella C, di Iorio G (2005) Increased cerebrospinal fluid levels of 3,3′,5′-triiodothyronine in patients with Alzheimer’s disease. J Clin Endocrinol Metab 90:198–202. https://doi.org/10.1210/jc.2004-1083
Yong-Hong L, Xiao-Dong P, Chang-Quan H et al (2013) Hypothalamic-pituitary-thyroid axis in patients with Alzheimer disease (AD). J Investig Med 61:578–581. https://doi.org/10.2310/JIM.0b013e318280aafb
Vargas JY, Ahumada J, Arrázola MS, Fuenzalida M, Inestrosa NC (2015) WASP-1, a canonical Wnt signaling potentiator, rescues hippocampal synaptic impairments induced by Aβ oligomers. Exp Neurol 264:14–25. https://doi.org/10.1016/j.expneurol.2014.11.005
Vargas JY, Fuenzalida M, Inestrosa NC (2014) In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci 34:2191–2202. https://doi.org/10.1523/JNEUROSCI.0862-13.2014
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11:77–86. https://doi.org/10.1038/nrn2755
Willert K, Nusse R (2012) Wnt proteins. Cold Spring Harb Perspect Biol 4:a007864. https://doi.org/10.1101/cshperspect.a007864
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4:68–75
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. https://doi.org/10.1016/j.devcel.2009.06.016
Varela-nallar L, Alfaro IE, Serrano FG et al (2010) Wingless-type family member 5A (Wnta-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A 107:10–15. https://doi.org/10.1073/pnas.1010011107 DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1010011107
Filippone MG, Di Palma T, Lucci V, Zannini M (2014) Pax8 modulates the expression of Wnt4 that is necessary for the maintenance of the epithelial phenotype of thyroid cells. BMC Mol Biol 15:21. https://doi.org/10.1186/1471-2199-15-21
Pasca di Magliano M, Di Lauro R, Zannini M (2000) Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci U S A 97:13144–13149. https://doi.org/10.1073/pnas.240336397
Miller LD, Park KS, Guo QM, Alkharouf NW, Malek RL, Lee NH, Liu ET, Cheng SY (2001) Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Mol Cell Biol 21:6626–6639. https://doi.org/10.1128/MCB.21.19.6626-6639.2001
Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng SY (2008) Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol 28:4598–4608. https://doi.org/10.1128/MCB.02192-07
O’Shea PJ, Kim DW, Logan JG et al (2012) Advanced bone formation in mice with a dominant-negative mutation in the thyroid hormone receptor β gene due to activation of Wnt/β-catenin protein signaling. J Biol Chem 287:17812–17822. https://doi.org/10.1074/jbc.M111.311464
Rosenfeld CS (2016) Sexspecific placental responses in fetal. Development 156:3422–3434. https://doi.org/10.1210/en.2015
Cvoro A, Devito L, Milton FA, Noli L, Zhang A, Filippi C, Sakai K, Suh JH et al (2015) A thyroid hormone receptor/KLF9 axis in human hepatocytes and pluripotent stem cells. Stem Cells 33:416–428. https://doi.org/10.1002/stem.1875
Chew L-J, Shen W, Ming X, Senatorov VV, Chen HL, Cheng Y, Hong E, Knoblach S et al (2011) SRY-box containing gene 17 regulates the Wnt/-catenin signaling pathway in oligodendrocyte progenitor cells. J Neurosci 31:13921–13935. https://doi.org/10.1523/JNEUROSCI.3343-11.2011
Acknowledgments
This work was supported by grants from the Basal Center of Excellence in Aging and Regeneration (CONICYT-AFB 170005) to N.C.I., FONDECYT (no. 1160724) to N.C.I., and FONDECYT (no. 11160651) to P.C. We also thank our special grant “Lithium in Health and Disease” from the Sociedad Química y Minera de Chile (SQM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Salazar, P., Cisternas, P., Martinez, M. et al. Hypothyroidism and Cognitive Disorders during Development and Adulthood: Implications in the Central Nervous System. Mol Neurobiol 56, 2952–2963 (2019). https://doi.org/10.1007/s12035-018-1270-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1270-y