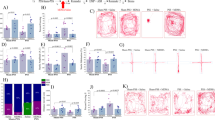
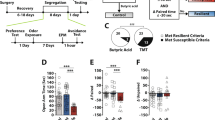
Abstract
The aim of the present study was to strengthen our hypothesis of a common physiological basis for post-traumatic stress disorder (PTSD) and substance use disorders. This paper investigates the possibility that rats exposed to a PTSD model exhibit noradrenergic and behavioral sensitization, as observed following repeated drugs of abuse injections. First, rats received a single prolonged stress (SPS), combining three consecutive stressors. They were then tested, 2 weeks after the trauma for PTSD-like symptoms to discriminate between vulnerable and resilient rats. When microdialysis was performed in the prelimbic cortex (Experiment 1), larger increases of noradrenaline (NA) release in response to amphetamine were observed in vulnerable rats when compared to control and resilient animals. Experiment 2 showed that trauma-vulnerable rats exhibited increases in locomotor activity relative to controls, in response to an exposure to trauma-associated cues. These data demonstrate that a single trauma exposure induces in vulnerable animals both, a noradrenergic sensitization evidenced within the prelimbic cortex and behavioral sensitization obtained after a physiologic activation of the noradrenergic system. However, Experiment 3 showed that when NA system was activated by amphetamine (1 mg/kg), a decrease in behavioral sensitization was obtained in vulnerable rats. We proposed that this decreased locomotor activity results from an additional stress-induced increased reactivity of mesocortical dopaminergic neurons, known to counteract the consequences of cortical noradrenergic release in rats. These results support our hypothesis that noradrenergic sensitization represents a common physiological basis, involved both in PTSD and drug addiction and suggest new common therapeutic approaches for these pathologies.



Similar content being viewed by others
References
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52:1048–1060
Gisquet-Verrier P, Tolédano D, Le Dorze C (2016) Bases physiologiques communes pour les troubles de stress post-traumatique et la dépendance aux drogues d’abus : Conséquences pour de nouvelles approches thérapeutiques. Therapie 72:357–366. https://doi.org/10.1016/j.therap.2016.07.005
Toledano D, Tassin J-P, Gisquet-Verrier P (2013) Traumatic stress in rats induces noradrenergic-dependent long-term behavioral sensitization: role of individual differences and similarities with dependence on drugs of abuse. Psychopharmacology 230:465–476. https://doi.org/10.1007/s00213-013-3179-5
Toledano D, Gisquet-Verrier P (2014) Only susceptible rats exposed to a model of PTSD exhibit reactivity to trauma-related cues and other symptoms: an effect abolished by a single amphetamine injection. Behav Brain Res 272:165–174. https://doi.org/10.1016/j.bbr.2014.06.039
Tassin J-P (2008) Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol 75:85–97. https://doi.org/10.1016/j.bcp.2007.06.038
Lanteri C, Hernández Vallejo SJ, Salomon L et al (2009) Inhibition of monoamine oxidases desensitizes 5-HT1A autoreceptors and allows nicotine to induce a neurochemical and behavioral sensitization. J Neurosci 29:987–997. https://doi.org/10.1523/JNEUROSCI.3315-08.2009
Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP (2008) Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology 33:1724–1734. https://doi.org/10.1038/sj.npp.1301548
Lanteri C, Doucet EL, Hernández Vallejo SJ, Godeheu G, Bobadilla AC, Salomon L, Lanfumey L, Tassin JP (2014) Repeated exposure to MDMA triggers long-term plasticity of noradrenergic and serotonergic neurons. Mol Psychiatry 19:823–833. https://doi.org/10.1038/mp.2013.97
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365. https://doi.org/10.1124/pr.109.001933
Antelman SM, Eichler AJ, Black CA, Kocan D (1980) Interchangeability of stress and amphetamine in sensitization. Science 207:329–331
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Barr AM, Hofmann CE, Weinberg J, Phillips AG (2002) Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology 26:286–294. https://doi.org/10.1016/S0893-133X(01)00308-6
Hamamura T, Fibiger HC (1993) Enhanced stress-induced dopamine release in the prefrontal cortex of amphetamine-sensitized rats. Eur J Pharmacol 237:65–71
Uban KA, Comeau WL, Bodnar T, Yu WK, Weinberg J, Galea LAM (2015) Amphetamine sensitization and cross-sensitization with acute restraint stress: impact of prenatal alcohol exposure in male and female rats. Psychopharmacology 232:1705–1716. https://doi.org/10.1007/s00213-014-3804-y
Quadir SG, dos Santos JRB, Campbell RR et al (2016) Homer2 regulates alcohol and stress cross-sensitization. Addict Biol 21:613–633. https://doi.org/10.1111/adb.12252
Nikulina EM, Covington HE, Ganschow L et al (2004) Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: fos in the ventral tegmental area and amygdala. Neuroscience 123:857–865
Cruz FC, Marin MT, Leão RM, Planeta CS (2012) Stress-induced cross-sensitization to amphetamine is related to changes in the dopaminergic system. J Neural Transm 119:415–424. https://doi.org/10.1007/s00702-011-0720-8
Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM (2013) Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens. Eur J Neurosci 37:982–995. https://doi.org/10.1111/ejn.12121
Liberzon I, Krstov M, Young EA (1997) Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22:443–453
Le Dorze C, Gisquet-Verrier P (2016) Sensitivity to trauma-associated cues is restricted to vulnerable traumatized rats and reinstated after extinction by yohimbine. Behav Brain Res 313:120–134. https://doi.org/10.1016/j.bbr.2016.07.006
Hinton DE, Pich V, Chhean D, Pollack MH, Barlow DH (2004) Olfactory-triggered panic attacks among Cambodian refugees attending a psychiatric clinic. Gen Hosp Psychiatry 26:390–397. https://doi.org/10.1016/j.genhosppsych.2004.04.007
Vermetten E, Schmahl C, Southwick SM, Bremner JD (2007) Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull 40:8–30
Le Dorze C, Gisquet-Verrier P (2016) Effects of multiple brief exposures to trauma-associated cues on traumatized resilient and vulnerable rats. Brain Res 1652:71–80. https://doi.org/10.1016/j.brainres.2016.10.002
Le Dorze C, Tassin J-P, Gisquet-verrier P (2016) Vulnerable traumatized rats demonstrate increases in medial prefrontal noradrenergic release: a support to the hypothesis of common physiological bases for PTSD and dependence to drugs of abuse. In: FENS - Copenhague (02–06 July)
Sara SJ (2000) Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7:73–84
Rouanet H, Bernard JM, Leroux B (1990) Statistiques en sciences humaines: analyse inductive des donnees, Dunod, Paris
Salomon L, Lanteri C, Glowinski J, Tassin J-P (2006) Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci U S A 103:7476–7481. https://doi.org/10.1073/pnas.0600839103
Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP (2002) Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci 22:2873–2884
Toledano D, Gisquet-Verrier P (2016) Repeated amphetamine injections alter behavior and induce a delayed behavioral sensitization modulated by reactivity to novelty: Similarities and differences with trauma consequences. Eur Neuropsychopharmacol 26:456–466. https://doi.org/10.1016/j.euroneuro.2015.12.042
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223. https://doi.org/10.1038/nrn2573
Doucet EL, Bobadilla A-C, Houades V, Lanteri C, Godeheu G, Lanfumey L, Sara SJ, Tassin JP (2013) Sustained impairment of α2A-adrenergic autoreceptor signaling mediates neurochemical and behavioral sensitization to amphetamine. Biol Psychiatry 74:90–98. https://doi.org/10.1016/j.biopsych.2012.11.029
Eagle AL, Singh R, Kohler RJ, Friedman AL, Liebowitz CP, Galloway MP, Enman NM, Jutkiewicz EM et al (2015) Single prolonged stress effects on sensitization to cocaine and cocaine self-administration in rats. Behav Brain Res 284:218–224. https://doi.org/10.1016/j.bbr.2015.02.027
Alttoa A, Eller M, Herm L, Rinken A, Harro J (2007) Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: Differences in the role of locus coeruleus. Brain Res 1131:138–148. https://doi.org/10.1016/j.brainres.2006.10.075
Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL (2009) Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav 96:493–504. https://doi.org/10.1016/j.physbeh.2008.12.001
Eagle AL, Perrine SA (2013) Methamphetamine-induced behavioral sensitization in a rodent model of posttraumatic stress disorder. Drug Alcohol Depend 131:36–43. https://doi.org/10.1016/j.drugalcdep.2013.04.001
Auclair A, Cotecchia S, Glowinski J, Tassin J-P (2002) D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci 22:9150–9154
Piazza PV, Rougé-Pont F, Deminière JM, Kharoubi M, le Moal M, Simon H (1991) Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res 567:169–174
Harvey BH, Brand L, Jeeva Z, Stein DJ (2006) Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav 87:881–890. https://doi.org/10.1016/j.physbeh.2006.01.033
Feenstra MG, Teske G, Botterblom MH, De Bruin JP (1999) Dopamine and noradrenaline release in the prefrontal cortex of rats during classical aversive and appetitive conditioning to a contextual stimulus: interference by novelty effects. Neurosci Lett 272:179–182
Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO (2005) Role of brain norepinephrine in the behavioral response to stress. Prog Neuro-Psychopharmacol Biol Psychiatry 29:1214–1224. https://doi.org/10.1016/j.pnpbp.2005.08.007
Blanc G, Hervé D, Simon H, Lisoprawski A, Glowinski J, Tassin JP (1980) Response to stress of mesocortico-frontal dopaminergic neurones in rats after long-term isolation. Nature 284:265–267
Thierry AM, Tassin JP, Blanc G, Glowinski J (1976) Selective activation of mesocortical DA system by stress. Nature 263:242–244
Gioanni Y, Thierry AM, Glowinski J, Tassin JP (1998) Alpha1-adrenergic, D1, and D2 receptors interactions in the prefrontal cortex: implications for the modality of action of different types of neuroleptics. Synapse 30:362–370. https://doi.org/10.1002/(SICI)1098-2396(199812)30:4<362::AID-SYN3>3.0.CO;2-W
Tassin J-P, Stinus L, Simon H et al (1978) Relationship between the locomotor hyperactivity induced by A10 lesions and the destruction of the frontocortical dopaminergic innervation in the rat. Brain Res 141:267–281
Polston JE, Glick SD (2011) Music-induced context preference following cocaine conditioning in rats. Behav Neurosci 125:674–680. https://doi.org/10.1037/a0024341
Trovero F, David S, Bernard P, Puech A, Bizot JC, Tassin JP (2016) The combination of marketed antagonists of α1b-adrenergic and 5-HT2A receptors inhibits behavioral sensitization and preference to alcohol in mice: a promising approach for the treatment of alcohol dependence. PLoS One 11:e0151242. https://doi.org/10.1371/journal.pone.0151242
Chopin M-V, Peretti C-S, Gisquet-Verrier P, Hoffmann C, Belaïd A, Chouinard G (2016) Cocaine use disorder treated with specific cognitive behavioral therapy and adjunctive propranolol. Psychother Psychosom 85:61–63. https://doi.org/10.1159/000441036
Acknowledgments
We are very grateful to Professor D. C. Riccio for the correction of the English text. We thank Aurelie Bonilla, Céline Dubois, and Fabien Lhéricel for the animal care.
Funding
This work was supported by “les Gueules Cassées” foundation (grant number 45-2017). Claire Le Dorze was supported by a DGA-MRIS (Direction Générale de l’Armement) scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 575 kb)
Rights and permissions
About this article
Cite this article
Le Dorze, C., Tassin, JP., Chauveau, F. et al. Behavioral and Noradrenergic Sensitizations in Vulnerable Traumatized Rats Suggest Common Bases with Substance Use Disorders. Mol Neurobiol 56, 611–620 (2019). https://doi.org/10.1007/s12035-018-1053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1053-5