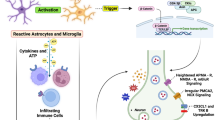
Abstract
Pain is a protective mechanism that enables us to avoid potentially harmful environments. However, when pathologically persisted and aggravated under severely injured or inflamed conditions, pain often reduces the quality of life and thus is considered as a disease to eliminate. Inflammatory and/or neuropathic mechanisms may exaggerate interactions between damaged tissues and neural pathways for pain mediation. Similar mechanisms also promote the communication among cellular participants in synapses at spinal or higher levels, which may amplify nociceptive firing and subsequent signal transmission, deteriorating the pain sensation. In this pathology, important cellular players are afferent sensory neurons, peripheral immune cells, and spinal glial cells. Arising from damage of injury, overloaded interstitial and intracellular reactive oxygen species (ROS) and intracellular Ca2+ are key messengers in the development and maintenance of pathologic pain. Thus, an ROS-sensitive and Ca2+-permeable ion channel that is highly expressed in the participant cells might play a critical role in the pathogenesis. Transient receptor potential melastatin subtype 2 (TRPM2) is the unique molecule that satisfies all of the requirements: the sensitivity, permeability, and its expressing cells. Notable progress in delineating the role of TRPM2 in pain has been achieved during the past decade. In the present review, we summarize the important findings in the key cellular components that are involved in pathologic pain. This overview will help to understand TRPM2-mediated pain mechanisms and speculate therapeutic strategies by utilizing this updated information.


Similar content being viewed by others
References
Linley JE, Rose K, Ooi L, Gamper N (2010) Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch 459(5):657–669. https://doi.org/10.1007/s00424-010-0784-6
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29(1):355–384. https://doi.org/10.1146/annurev-cellbio-101011-155833
Chiu IM, von Hehn CA, Woolf CJ (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15(8):1063–1067. https://doi.org/10.1038/nn.3144
Pinho-Ribeiro FA, Verri WA Jr, Chiu IM (2017) Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 38(1):5–19. https://doi.org/10.1016/j.it.2016.10.001
Zhang ZJ, Jiang BC, Gao YJ (2017) Chemokines in neuron–glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 74(18):3275–3291. https://doi.org/10.1007/s00018-017-2513-1
Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, Watkins LR (2016) Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci 39(12):862–879. https://doi.org/10.1016/j.tins.2016.10.003
Gamper N, Ooi L (2015) Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid Redox Signal 22(6):486–504. https://doi.org/10.1089/ars.2014.5884
Choi SI, Yoo S, Lim JY, Hwang SW (2014) Are sensory TRP channels biological alarms for lipid peroxidation? Int J Mol Sci 15(9):16430–16457. https://doi.org/10.3390/ijms150916430
Binder CJ, Papac-Milicevic N, Witztum JL (2016) Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol 16(8):485–497. https://doi.org/10.1038/nri.2016.63
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50(4):264–274. https://doi.org/10.1038/sc.2011.111
Fatima G, Sharma VP, Das SK, Mahdi AA (2015) Oxidative stress and antioxidative parameters in patients with spinal cord injury: implications in the pathogenesis of disease. Spinal Cord 53(1):3–6. https://doi.org/10.1038/sc.2014.178
Oates PJ (2008) Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets 9(1):14–36. https://doi.org/10.2174/138945008783431781
Sozbir E, Naziroglu M (2016) Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab Brain Dis 31(2):385–393. https://doi.org/10.1007/s11011-015-9769-7
Faouzi M, Penner R (2014) Trpm2. Handb Exp Pharmacol 222:403–426. https://doi.org/10.1007/978-3-642-54215-2_16
Jiang LH, Yang W, Zou J, Beech DJ (2010) TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets 14(9):973–988. https://doi.org/10.1517/14728222.2010.510135
Vandewauw I, Owsianik G, Voets T (2013) Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci 14(1):21. https://doi.org/10.1186/1471-2202-14-21
Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, Shirakawa H, Mori Y et al (2012) TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci 32(11):3931–3941. https://doi.org/10.1523/JNEUROSCI.4703-11.2012
Syed Mortadza SA, Wang L, Li D, Jiang LH (2015) TRPM2 channel-mediated ROS-sensitive Ca(2+) signaling mechanisms in immune cells. Front Immunol 6:407
Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N (1998) Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54(1):124–131. https://doi.org/10.1006/geno.1998.5551
Naziroglu M, Ozgul C, Celik O, Cig B, Sozbir E (2011) Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J Membr Biol 241(2):69–75. https://doi.org/10.1007/s00232-011-9363-9
Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25(9):1804–1815. https://doi.org/10.1038/sj.emboj.7601083
Kolisek M, Beck A, Fleig A, Penner R (2005) Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 18(1):61–69. https://doi.org/10.1016/j.molcel.2005.02.033
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q et al (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411(6837):595–599. https://doi.org/10.1038/35079100
Lund FE (2006) Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med 12(11–12):328–333. https://doi.org/10.2119/2006-00099.Lund
Long A, Klimova N, Kristian T (2017) Mitochondrial NUDIX hydrolases: a metabolic link between NAD catabolism. GTP and mitochondrial dynamics, Neurochem Int
Min W, Wang ZQ (2009) Poly (ADP-ribose) glycohydrolase (PARG) and its therapeutic potential. Front Biosci (Landmark Ed) 14:1619–1626
Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K (2001) Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293(5533):1327–1330. https://doi.org/10.1126/science.1062473
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S et al (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9(1):163–173. https://doi.org/10.1016/S1097-2765(01)00438-5
Ge Y, Jiang W, Gan L, Wang L, Sun C, Ni P, Liu Y, Wu S et al (2010) Mouse embryonic fibroblasts from CD38 knockout mice are resistant to oxidative stresses through inhibition of reactive oxygen species production and Ca(2+) overload. Biochem Biophys Res Commun 399(2):167–172. https://doi.org/10.1016/j.bbrc.2010.07.040
Ma Y, Nie H, Chen H, Li J, Hong Y, Wang B, Wang C, Zhang J et al (2015) NAD(+)/NADH metabolism and NAD(+)-dependent enzymes in cell death and ischemic brain injury: current advances and therapeutic implications. Curr Med Chem 22(10):1239–1247. https://doi.org/10.2174/0929867322666150209154420
Di Meglio S, Tramontano F, Cimmino G, Jones R, Quesada P (2004) Dual role for poly(ADP-ribose)polymerase-1 and -2 and poly(ADP-ribose)glycohydrolase as DNA-repair and pro-apoptotic factors in rat germinal cells exposed to nitric oxide donors. Biochim Biophys Acta 1692(1):35–44. https://doi.org/10.1016/j.bbamcr.2004.04.002
Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D et al (2005) Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280(7):6138–6148. https://doi.org/10.1074/jbc.M411446200
Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277(26):23150–23156. https://doi.org/10.1074/jbc.M112096200
Kuhn FJ, Luckhoff A (2004) Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J Biol Chem 279(45):46431–46437. https://doi.org/10.1074/jbc.M407263200
Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD et al (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 143(1):186–192. https://doi.org/10.1038/sj.bjp.0705914
Buelow B, Song Y, Scharenberg AM (2008) The poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem 283(36):24571–24583. https://doi.org/10.1074/jbc.M802673200
Kashio M, Sokabe T, Shintaku K, Uematsu T, Fukuta N, Kobayashi N, Mori Y, Tominaga M (2012) Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci U S A 109(17):6745–6750. https://doi.org/10.1073/pnas.1114193109
Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J (2016) The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353(6306):1393–1398. https://doi.org/10.1126/science.aaf7537
Tan CH, McNaughton PA (2016) The TRPM2 ion channel is required for sensitivity to warmth. Nature 536(7617):460–463. https://doi.org/10.1038/nature19074
Le Pichon CE, Chesler AT (2014) The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 8:21
Raible DW, Ungos JM (2006) Specification of sensory neuron cell fate from the neural crest. Adv Exp Med Biol 589:170–180. https://doi.org/10.1007/978-0-387-46954-6_10
Yoo S, Lim JY, Hwang SW (2014) Sensory TRP channel interactions with endogenous lipids and their biological outcomes. Molecules 19(4):4708–4744. https://doi.org/10.3390/molecules19044708
Matsumoto K, Takagi K, Kato A, Ishibashi T, Mori Y, Tashima K, Mitsumoto A, Kato S et al (2016) Role of transient receptor potential melastatin 2 (TRPM2) channels in visceral nociception and hypersensitivity. Exp Neurol 285(Pt a):41–50
Naziroglu M, Ozgul C, Cig B, Dogan S, Uguz AC (2011) Glutathione modulates Ca(2+) influx and oxidative toxicity through TRPM2 channel in rat dorsal root ganglion neurons. J Membr Biol 242(3):109–118. https://doi.org/10.1007/s00232-011-9382-6
Naziroglu M, Uguz AC, Ismailoglu O, Cig B, Ozgul C, Borcak M (2013) Role of TRPM2 cation channels in dorsal root ganglion of rats after experimental spinal cord injury. Muscle Nerve 48(6):945–950. https://doi.org/10.1002/mus.23844
Naziroglu M, Ozgul C (2013) Vitamin E modulates oxidative stress and protein kinase C activator (PMA)-induced TRPM2 channel gate in dorsal root ganglion of rats. J Bioenerg Biomembr 45(6):541–549. https://doi.org/10.1007/s10863-013-9524-x
Naziroglu M (2017) Activation of TRPM2 and TRPV1 channels in dorsal root ganglion by NADPH oxidase and protein kinase C molecular pathways: a patch clamp study. J Mol Neurosci 61(3):425–435. https://doi.org/10.1007/s12031-017-0882-4
Naziroglu M, Cig B, Ozgul C (2013) Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242:151–160. https://doi.org/10.1016/j.neuroscience.2013.03.032
Chung MK, Asgar J, Lee J, Shim MS, Dumler C, Ro JY (2015) The role of TRPM2 in hydrogen peroxide-induced expression of inflammatory cytokine and chemokine in rat trigeminal ganglia. Neuroscience 297:160–169. https://doi.org/10.1016/j.neuroscience.2015.03.067
Silva RL, Lopes AH, Guimaraes RM, Cunha TM (2017) CXCL1/CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol Dis 105:109–116. https://doi.org/10.1016/j.nbd.2017.06.001
Miller RJ, Jung H, Bhangoo SK, White FA (2009) Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 194:417–449. https://doi.org/10.1007/978-3-540-79090-7_12
Steinberg SF (2015) Mechanisms for redox-regulation of protein kinase C. Front Pharmacol 6:128
Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR (2012) Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci 13(9):10697–10721. https://doi.org/10.3390/ijms130910697
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66(8):1499–1503. https://doi.org/10.1016/S0006-2952(03)00504-5
Ren K, Dubner R (2010) Interactions between the immune and nervous systems in pain. Nat Med 16(11):1267–1276. https://doi.org/10.1038/nm.2234
Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T et al (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14(7):738–747. https://doi.org/10.1038/nm1758
Wehrhahn J, Kraft R, Harteneck C, Hauschildt S (2010) Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol 184(5):2386–2393. https://doi.org/10.4049/jimmunol.0902474
Gelderblom M, Melzer N, Schattling B, Gob E, Hicking G, Arunachalam P, Bittner S, Ufer F et al (2014) Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke 45(11):3395–3402. https://doi.org/10.1161/STROKEAHA.114.005836
Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, Mori Y, Shimizu S (2013) Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc Res 97(2):271–281. https://doi.org/10.1093/cvr/cvs332
Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L (2013) TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun 4:1611. https://doi.org/10.1038/ncomms2608
Qian X, Numata T, Zhang K, Li C, Hou J, Mori Y, Fang X (2014) Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology 121(2):336–351. https://doi.org/10.1097/ALN.0000000000000275
Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM et al (2011) The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 13(1):29–34. https://doi.org/10.1038/ni.2171
Hwang SW, Oh U (2007) Current concepts of nociception: nociceptive molecular sensors in sensory neurons. Curr Opin Anaesthesiol 20(5):427–434. https://doi.org/10.1097/ACO.0b013e3282eff91c
So K, Haraguchi K, Asakura K, Isami K, Sakimoto S, Shirakawa H, Mori Y, Nakagawa T et al (2015) Involvement of TRPM2 in a wide range of inflammatory and neuropathic pain mouse models. J Pharmacol Sci 127(3):237–243. https://doi.org/10.1016/j.jphs.2014.10.003
Blackshaw LA, Brierley SM, Hughes PA (2010) TRP channels: new targets for visceral pain. Gut 59(1):126–135. https://doi.org/10.1136/gut.2009.179523
Balemans D, Boeckxstaens GE, Talavera K, Wouters MM (2017) Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 312(6):G635–G648. https://doi.org/10.1152/ajpgi.00401.2016
Fuentes IM, Christianson JA (2016) Ion channels, ion channel receptors, and visceral hypersensitivity in irritable bowel syndrome. Neurogastroenterol Motil 28(11):1613–1618. https://doi.org/10.1111/nmo.12979
Wu CL, Raja SN (2011) Treatment of acute postoperative pain. Lancet 377(9784):2215–2225
Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ et al (2009) Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology 110(1):155–165. https://doi.org/10.1097/ALN.0b013e318190bc16
Ozdemir US, Naziroglu M, Senol N, Ghazizadeh V (2016) Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 53(6):3540–3551. https://doi.org/10.1007/s12035-015-9292-1
Uslusoy F, Naziroglu M, Cig B (2017) Inhibition of the TRPM2 and TRPV1 channels through Hypericum perforatum in sciatic nerve injury-induced rats demonstrates their key role in apoptosis and mitochondrial oxidative stress of sciatic nerve and dorsal root ganglion. Front Physiol 8:335
Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA (2002) Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain 100(1–2):163–170. https://doi.org/10.1016/S0304-3959(02)00257-9
Hu P, Bembrick AL, Keay KA, McLachlan EM (2007) Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 21(5):599–616. https://doi.org/10.1016/j.bbi.2006.10.013
Isami K, Haraguchi K, So K, Asakura K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S (2013) Involvement of TRPM2 in peripheral nerve injury-induced infiltration of peripheral immune cells into the spinal cord in mouse neuropathic pain model. PLoS One 8(7):e66410. https://doi.org/10.1371/journal.pone.0066410
Feldman EL, Nave KA, Jensen TS, Bennett DL (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93(6):1296–1313. https://doi.org/10.1016/j.neuron.2017.02.005
Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL (2013) Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol 216(1):1–11. https://doi.org/10.1530/JOE-12-0356
Kahya MC, Naziroglu M, Ovey IS (2017) Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol 54(3):2345–2360. https://doi.org/10.1007/s12035-016-9727-3
Pitcher T, Sousa-Valente J, Malcangio M (2016) The monoiodoacetate model of osteoarthritis pain in the mouse. J Vis Exp 111
Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, Im HJ (2013) A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527(2):440–447. https://doi.org/10.1016/j.gene.2013.05.069
Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM (2012) CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 109(50):20602–20607. https://doi.org/10.1073/pnas.1209294110
Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, Kendall DA, Scammell BE et al (2011) The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol Pain 7:88
Thakur M, Dickenson AH, Baron R (2014) Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol 10(6):374–380. https://doi.org/10.1038/nrrheum.2014.47
Flatters SJ, Bennett GJ (2006) Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122(3):245–257. https://doi.org/10.1016/j.pain.2006.01.037
Jaggi AS, Singh N (2012) Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 291(1–3):1–9. https://doi.org/10.1016/j.tox.2011.10.019
Miller SD, Karpus WJ, Davidson TS (2010) Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol chapter 15:Unit 15 11
Melzer N, Hicking G, Gobel K, Wiendl H (2012) TRPM2 cation channels modulate T cell effector functions and contribute to autoimmune CNS inflammation. PLoS One 7(10):e47617. https://doi.org/10.1371/journal.pone.0047617
Jang Y, Lee MH, Lee J, Jung J, Lee SH, Yang DJ, Kim BW, Son H et al (2014) TRPM2 mediates the lysophosphatidic acid-induced neurite retraction in the developing brain. Pflugers Arch 466(10):1987–1998. https://doi.org/10.1007/s00424-013-1436-4
Jang Y, Lee SH, Lee B, Jung S, Khalid A, Uchida K, Tominaga M, Jeon D et al (2015) TRPM2, a susceptibility gene for bipolar disorder, regulates glycogen synthase kinase-3 activity in the brain. J Neurosci 35(34):11811–11823. https://doi.org/10.1523/JNEUROSCI.5251-14.2015
Xie YF, Macdonald JF, Jackson MF (2010) TRPM2, calcium and neurodegenerative diseases. Int J Physiol Pathophysiol Pharmacol 2(2):95–103
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2017R1A2B2001817 and 2017M3C7A1025600). The authors declare that there is no conflict of interest regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jang, Y., Cho, P.S., Yang, Y.D. et al. Nociceptive Roles of TRPM2 Ion Channel in Pathologic Pain. Mol Neurobiol 55, 6589–6600 (2018). https://doi.org/10.1007/s12035-017-0862-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0862-2