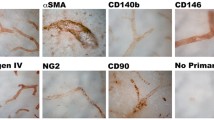
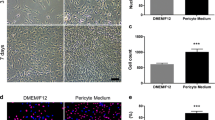
Abstract
While pericytes wrap around microvascular endothelial cells throughout the human body, their highest coverage rate is found in the brain. Brain pericytes actively contribute to various brain functions, including the development and stabilization of the blood-brain barrier (BBB), tissue regeneration, and brain inflammation. Accordingly, detailed characterization of the functional nature of brain pericytes is important for understanding the mechanistic basis of brain physiology and pathophysiology. Herein, we report on the development of a new human brain pericyte cell line, hereafter referred to as the human brain pericyte/conditionally immortalized clone 37 (HBPC/ci37). Developed via the cell conditionally immortalization method, these cells exhibited excellent proliferative ability at 33 °C. However, when cultured at 37 °C, HBPC/ci37 cells showed a differentiated phenotype that was marked by morphological alterations and increases in several pericyte-enriched marker mRNA levels, such as platelet-derived growth factor receptor β. It was also found that HBPC/ci37 cells possessed the facilitative ability of in vitro BBB formation and differentiation into a neuronal lineage. Furthermore, HBPC/ci37 cells exhibited the typical “reactive” features of brain pericytes in response to pro-inflammatory cytokines. To summarize, our results clearly demonstrate that HBPC/ci37 cells possess the ability to perform several key brain pericyte functions while also showing the capacity for extensive and continuous proliferation. Based on these findings, it can be expected that, as a unique human brain pericyte model, HBPC/ci37 cells have the potential to contribute to significant advances in the understanding of human brain pericyte physiology and pathophysiology.







Similar content being viewed by others
References
Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T (2016) What is a pericytes? J Cereb Blood Flow Metab 36:451–455
Sá-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45:327–347
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 7:452–464
Frank RN, Dutta S, Mancini MA (1987) Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci 28:1086–1091
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7:1031–1038
Dore-Duffy P, Katychev A, Wang X, Van Buren E (2006) CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 26:613–624
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM et al (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55–60
Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat Neurosci 19:771–783
Kisler K, Nelson AR, Montagne A, Zlokovic BV (2017) Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18:419–434
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468:562–566
Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M (2009) A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 54:253–263
Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ (2011) Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 199:223–229
Dore-Duffy P (2008) Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 14:1581–1593
Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA et al (2012) Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 11:471–476
Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K et al (2012) The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One 7:e35577
Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A et al (2015) Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 33:1962–1974
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738
Van de Haar HJ, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Wong SM, Hofman PA, Burgmans S et al (2016) Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging 45:190–196
Dore-Duffy P, Cleary K (2011) Morphology and properties of pericytes. Methods Mol Biol 686:49–68
Kovac A, Erickson MA, Banks WA (2011) Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation 8:139
Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, Mee EW, Faull RL et al (2014) A role for human brain pericytes in neuroinflammation. J Neuroinflammation 11:104
Rustenoven J, Jansson D, Smyth LC, Dragunow M (2016) Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 38:291–304
Ide T (2006) Mechanism of cell proliferation—cell cycle, oncogenes, and senescence. Yakugaku Zasshi 126:1087–1115
Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L et al (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13:630–638
Tabuchi Y, Arai Y, Ohta S, Shioya H, Takahashi R, Ueda M, Takeguchi N, Asano S et al (2002) Development and characterization of conditionally immortalized gastric epithelial cell lines from transgenic rats harboring temperature-sensitive simian virus 40 large T-antigen gene. Cell Struct Funct 27:71–79
Kamiichi A, Furihata T, Kishida S, Ohta Y, Saito K, Kawamatsu S, Chiba K (2012) Establishment of a new conditionally immortalized cell line from human brain microvascular endothelial cells: a promising tool for human blood-brain barrier studies. Brain Res 1488:113–122
Kowolik CM, Liang S, Yu Y, Yee JK (2004) Cre-mediated reversible immortalization of human renal proximal tubular epithelial cells. Oncogene 23:5950–5957
O’Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR et al (2001) Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci U S A 98:646–651
Sharma GG, Gupta A, Wang H, Scherthan H, Dhar S, Gandhi V, Iliakis G, Shay JW et al (2003) hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 22:131–146
Furihata T, Ito R, Kamiichi A, Saito K, Chiba K (2016) Establishment and characterization of a new conditionally immortalized human astrocyte cell line. J Neurochem 136:92–105
Kohno S, Murata T, Koide N, Hikita K, Kaneda N (2006) Establishment and characterization of a noradrenergic adrenal chromaffin cell line, tsAM5NE, immortalized with the temperature-sensitive SV40 T-antigen. Cell Biol Int 35:325–334
Hotta Y, Kaneko K, Inuma J, Inami Y, Aruga S, Shimaoka T, Sekiguchi Y, Io H et al (2010) Establishment of a peritoneal mesothelial cell line from a transgenic rat harboring the temperature-sensitive simian virus 40 large T-antigen gene. Nephrol Dial Transplant 25:1825–1832
Sarrab RM, Lennon R, Ni L, Wherlock MD, Welsh GI, Saleem MA (2011) Establishment of conditionally immortalized human glomerular mesangial cells in culture, with unique migratory properties. Am J Physiol Renal Physiol 301:1131–1138
Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ (2014) Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res 1550:1–8
Fernández-Klett F, Priller J (2015) Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab 35:883–887
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443:700–704
Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U (2010) Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 107:22290–22295
Wu T, Huang J, Moore PJ, Little MS, Walton WG, Fellner RC, Alexis NE, Peter Di Y et al (2017) Identification of BPIFA1/SPLUNC1 as an epithelium-derived smooth muscle relaxing factor. Nat Commun 8:14118
Chen PY, Qin L, Li G, Tellides G, Simons M (2016) Smooth muscle FGF/TGFβ cross talk regulates atherosclerosis progression. EMBO Mol Med 8:712–728
Neuhaus AA, Couch Y, Sutherland BA, Buchan AM (2017) Novel method to study pericyte contractility and responses to ischaemia in vitro using electrical impedance. J Blood Flow Metab 37:2013–2024
Persidsky Y, Hill J, Zhang M, Dykstra H, Winfield M, Reichenbach NL, Potula R, Mukherjee A et al (2016) Dysfunction of brain pericytes in chronic neuroinflammation. J Blood Flow Metab 36:794–807
Van Dijk CG, Nieuweboer FE, Pei JY, YJ X, Burgisser P, van Mulligen E, el Azzouzi H, Duncker DJ et al (2015) The complex mural cell: pericyte function in health and disease. Int J Cardiol 190:75–89
Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellström M, Lindahl P, Betsholtz C (2006) Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20:1703–1705
Ma SH, Lepak LA, Hussain RJ, Shain W, Shuler ML (2005) An endothelial and astrocyte co-culture model of the blood-brain barrier utilizing an ultra-thin, nanofabricated silicon nitride membrane. Lab Chip 5:74–85
Ozen I, Boix J, Paul G (2012) Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clin Transl Med 1:30
Fernández-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, Engel O, Stenzel W et al (2013) Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 33:428–439
Wang K, Bekar LK, Furber K, Walz W (2004) Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res 1024:193–202
Gelareh M, Leavitt BR (2015) Indoleamine 2,3 dioxygenase as a potential therapeutic target in Huntington’s disease. J Huntingt Dis 4:109–118
Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE et al (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302
Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV (2013) Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4:2932
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2015) Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 128:81–93
Acknowledgements
We would like to thank Dr. Mari Hashimoto (Research Center for Molecular Medicine of the Austrian Academy of Sciences) for providing the technical support to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethics Committee of the Chiba University (Chiba, Japan) approved the use of human cells in this study.
Rights and permissions
About this article
Cite this article
Umehara, K., Sun, Y., Hiura, S. et al. A New Conditionally Immortalized Human Fetal Brain Pericyte Cell Line: Establishment and Functional Characterization as a Promising Tool for Human Brain Pericyte Studies. Mol Neurobiol 55, 5993–6006 (2018). https://doi.org/10.1007/s12035-017-0815-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0815-9