Abstract
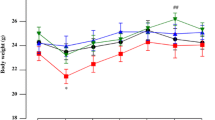
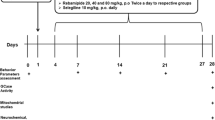
Elevated levels of acrolein, an α,β-unsaturated aldehyde are detected in the brain of patients with Parkinson’s disease (PD). In the present study, the neuroprotective effect of baicalein (a phenolic flavonoid in the dried root of Scutellaria baicalensis Georgi) on acrolein-induced neurodegeneration of nigrostriatal dopaminergic system was investigated using local infusion of acrolein in the substantia nigra (SN) of rat brain. Systemic administration of baicalein (30 mg/kg, i.p.) significantly attenuated acrolein-induced elevations in 4-hydroxy-2-noneal (a product of lipid peroxidation), N-(3-formyl-3,4-dehydropiperidino)lysine (a biomarker of acrolein-conjugated proteins), and heme-oxygenase-1 levels (a redox-regulated protein) in the infused SN, indicating that baicalein inhibited acrolein-induced oxidative stress and protein conjugation. Furthermore, baicalein reduced acrolein-induced elevations in glial fibrillary acidic protein (a biomarker of activated astrocytes), ED-1 (a biomarker of activated microglia), and mature cathepsin B levels (a cysteine lysosomal protease), suggesting that baicalein attenuated acrolein-induced neuroinflammation. Moreover, baicalein attenuated acrolein-induced caspase 1 activation (a pro-inflammatory caspase) and interleukin-1β levels, indicating that baicalein prevented acrolein-induced inflammasome activation. In addition, baicalein significantly attenuated acrolein-induced caspase 3 activation (a biomarker of apoptosis) as well as acrolein-induced elevation in receptor interacting protein kinase (RIPK) 3 levels (an initiator of necroptosis), indicating that baicalein attenuated apoptosis and necroptosis. At the same time, baicalein mitigated acrolein-induced reduction in dopamine levels in the striatum ipsilateral to acrolein-infused SN. In conclusion, our data suggest that baicalein is neuroprotective via inhibiting oxidative stress, protein conjugation, and inflammation. Furthermore, baicalein prevents acrolein-induced program cell deaths, suggesting that baicalein is therapeutically useful for slowing PD progression.




Similar content being viewed by others
References
de Carvalho RS, Duarte FS, de Lima TC (2011) Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav Brain Res 221:75–82
Cui L, Zhang X, Yang R, Liu L, Wang L et al (2010) Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav 96:469–475
Cheng Y, He G, Mu X, Zhang T, Li X et al (2008) Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett 441:16–20
Mu X, He G, Cheng Y, Li X, Xu B et al (2009) Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav 92:642–648
Choi JH, Choi AY, Yoon H, Choe W, Yoon KS et al (2010) Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Exp Mol Med 42:811–822
Li YC, Lin HJ, Yang JH, Yang JS, Ho HC et al (2009) Baicalein-induced apoptosis via endoplasmic reticulum stress through elevations of reactive oxygen species and mitochondria dependent pathway in mouse-rat hybrid retina ganglion cells (N18). Neurochem Res 34:418–429
Hung KC, Huang HJ, Wang YT, Lin AM (2016) Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the MPP+-treated nigrostriatal dopaminergic system in vivo. J Ethnopharmacol 194:522–529
Pang H, Xue W, Shi A, Li M, Li Y et al (2016) Multiple-ascending-dose pharmacokinetics and safety evaluation of baicalein chewable tablets in healthy Chinese volunteers. Clin Drug Investig 36:713–724
Yu X, He G, Du G (2012) Neuroprotective effect of baicalein in patients with Parkinson’s disease. Zhongguo Zhong Yao Za Zhi 37:421–425
Shamoto-Nagai M, Maruyama W, Hashizume Y, Yoshida M, Osawa T et al (2007) In parkinsonian substantia nigra, alpha-synuclein is modified by acrolein, a lipid-peroxidation product, and accumulates in the dopamine neurons with inhibition of proteasome activity. J Neural Transm (Vienna) 114:1559–1567
Park J, Muratori B, Shi R (2014) Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury. Neural Regen Res 9:677–683
Uchida K (1999) Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med 9:109–113
Adams JD Jr, Klaidman LK (1993) Acrolein-induced oxygen radical formation. Free Radic Biol Med 15:187–193
Luo J, Robinson JP, Shi R (2005) Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int 47:449–457
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Jeong MS, Kang JH (2008) Acrolein, the toxic endogenous aldehyde, induces neurofilament-L aggregation. BMB Rep 41:635–639
Lovell MA, Xie C, Markesbery WR (2001) Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 22:187–194
Liu-Snyder P, McNally H, Shi R, Borgens RB (2006) Acrolein-mediated mechanisms of neuronal death. J Neurosci Res 84:209–218
Due MR, Park J, Zheng L, Walls M, Allette YM et al (2014) Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem 128:776–786
Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA (2001) Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int 39:141–149
Schaur RJ, Siems W, Bresgen N, Eckl PM (2015) 4-Hydroxy-nonenal—a bioactive lipid peroxidation product. Biomol Ther 5:2247–2337
Wu CC, Hsieh CW, Lai PH, Lin JB, Liu YC et al (2006) Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol Appl Pharmacol 214:244–252
Chiueh CC, Zukowska-Grojec Z, Kirk KL, Kopin IJ (1983) 6-Fluorocatecholamines as false adrenergic neurotransmitters. J Pharmacol Exp Ther 225:529–533
Yokoyama H, Kuroiwa H, Yano R, Araki T (2008) Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson’s disease. Neurol Sci 29:293–301
Beraud D, Hathaway HA, Trecki J, Chasovskikh S, Johnson DA et al (2013) Microglial activation and antioxidant responses induced by the Parkinson’s disease protein alpha-synuclein. J NeuroImmune Pharmacol 8:94–117
Sharma N, Nehru B (2015) Characterization of the lipopolysaccharide induced model of Parkinson’s disease: role of oxidative stress and neuroinflammation. Neurochem Int 87:92–105
Spencer JP, Vafeiadou K, Williams RJ, Vauzour D (2012) Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Asp Med 33:83–97
Lee E, Park HR, Ji ST, Lee Y, Lee J (2014) Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J Neurosci Res 92:130–139
Brown GC, Vilalta A (2015) How microglia kill neurons. Brain Res 1628:288–297
Nakanishi H (2003) Microglial functions and proteases. Mol Neurobiol 27:163–176
Lu KT, Wang YW, Yang JT, Yang YL, Chen HI (2005) Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma 22:885–895
Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD (2014) Caspase-1: the inflammasome and beyond. Innate Immun 20:115–125
Tanel A, Averill-Bates DA (2005) The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim Biophys Acta 1743:255–267
Dong L, Zhou S, Yang X, Chen Q, He Y et al (2013) Magnolol protects against oxidative stress-mediated neural cell damage by modulating mitochondrial dysfunction and PI3K/Akt signaling. J Mol Neurosci 50:469–481
Liu C, Wu J, Xu K, Cai F, Gu J et al (2010) Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem 112:1500–1512
Abu-Qare AW, Abou-Donia MB (2001) Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J Toxicol Environ Health B Crit Rev 4:313–332
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517:311–320
Zhou W, Yuan J (2014) Necroptosis in health and diseases. Semin Cell Dev Biol 35:14–23
Chan FK, Luz NF, Moriwaki K (2015) Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol 33:79–106
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The use of animals has been approved by the Institutional Animal Care and Use Committee of Taipei Veterans General Hospital, Taipei, Taiwan, R.O.C. All experiments were performed in accordance with the approved guidelines. The approval number is IACUC2014-186.
Rights and permissions
About this article
Cite this article
Zhao, WZ., Wang, HT., Huang, HJ. et al. Neuroprotective Effects of Baicalein on Acrolein-induced Neurotoxicity in the Nigrostriatal Dopaminergic System of Rat Brain. Mol Neurobiol 55, 130–137 (2018). https://doi.org/10.1007/s12035-017-0725-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0725-x