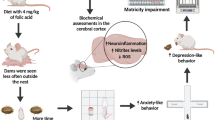
Abstract
Although folic acid (FA) supplementation is known to influence numerous physiological functions, especially during pregnancy, little is known about its direct effects on the mothers’ health. However, this vitamin is essential for the health of the mother and for the normal growth and development of the fetus. Thus, the aim of this study was (1) to evaluate the cognitive effects and biochemical markers produced by the AIN-93 diet (control), the AIN-93 diet supplemented with different doses of FA (5, 10, and 50 mg/kg), and a FA-deficient diet during pregnancy and lactation in female mother rats (dams) and (2) to evaluate the effect of maternal diets on inflammatory parameters in the adult offspring which were subjected to an animal model of schizophrenia (SZ) induced by ketamine (Ket). Our study demonstrated through the Y-maze test that rats subjected to the FA-deficient diet showed significant deficits in spatial memory, while animals supplemented with FA (5 and 10 mg/kg) showed no deficit in spatial memory. Our results also suggest that the rats subjected to the FA-deficient diet had increased levels of carbonylated proteins in the frontal cortex and hippocampus and also increased plasma levels of homocysteine (Hcy). Folate was able to prevent cognitive impairments in the rats supplemented with FA (5 and 10 mg/kg), data which may be attributed to the antioxidant effect of the vitamin. Moreover, FA prevented protein damage and elevations in Hcy levels in the rats subjected to different doses of this vitamin (5, 10, and 50 mg/kg). We verified a significant increase of the anti-inflammatory cytokine (interleukin-4 (IL-4)) and a reduction in the plasma levels of proinflammatory cytokines (interleukin-6 (IL-6)) and TNF-α) in the dams that were subjected to the diets supplemented with FA (5, 10, and 50 mg/kg), showing the possible anti-inflammatory effects of FA during pregnancy and lactation. In general, we also found that in the adult offspring that were subjected to an animal model of SZ, FA had a protective effect in relation to the levels of IL-4, IL-6, and TNF-α, which indicates that the action of FA persisted in the adult offspring, since FA showed a lasting effect on the inflammatory response, which was similar in both the dams and their offspring. In conclusion, the importance of supplementation with FA during pregnancy and lactation should be emphasized, not only for the benefit of the offspring but also for the health of the mother. All this is due to the considerable protective effect of this vitamin against oxidative damage, cognitive impairment, hyperhomocysteinemia, immune function, and also its ability in preventing common processes in post-pregnancy stages, as well as in reducing the risks of neurodevelopmental disorders and enhancing fetal immune development.









Similar content being viewed by others
Abbreviations
- FA:
-
folic acid
- Dams:
-
Female mother rat
- Hcy:
-
Homocysteine
- HHcy:
-
Hiperhomocisteinemia
- NMDA:
-
N-methyl-d-aspartate
- SAH, SAM:
-
S-adenosyl-methionine
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system (CNS)
- SZ:
-
Schizophrenia
- TBARS:
-
Thiobarbituric acid reactive species
- MAD:
-
Malondialdehyde
- DNPH:
-
Dinitrophenylhydrazine
- EDTA:
-
Ethylenediaminetetraacetic acid
References
Darnton-Hill I, Mkparu UC (2015) Micronutrients in pregnancy in low- and middle-income countries. Nutrients 7(3):1744–1768
Berti C, Biesalski HK, Gärtner R, Lapillone A, Pietrzik K, Poston L, Redman C, Koletzko B et al (2011) Micronutrients in pregnancy: current knowledge and unresolved questions. Clin Nutr 30(6):689–701
Darnton-Hill I (2012) Global burden and significance of multiple micronutrient deficiencies in pregnancy. Nestle Nutr Inst Workshop Ser 70:49–60
Raiten DJ, Namasté S, Brabin B, Combs G, L’Abbé MR, Wasantwisut E, Darnton-Hill I (2011) Executive summary: biomarkers of nutrition for development (BOND): building a consensus. Am J Clin Nutr 94:633S–650S
Wallingford JB, Niswander LA, Shaw GM, Finnell RH (2013) The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339(6123):1222002
Stamm RA, Houghton LA (2013) Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 5:3920–3947
Miller AL, Kelly GS (1996) Methionine and homocysteine metabolism and the nutritional prevention of certain birth defects and complications of pregnancy. Alt Med Rev 1(4):220–235
Micle O, Muresan M, Antal L, Bodog F, Bodog A (2012) The influence of homocysteine and oxidative stress on pregnancy outcome. J Med Life 5(1):68–73
White AR, Huang X, Jobling MF, Barrow CJ, Beyreuther K, Masters CL, Bush AL, Cappai R (2001) Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J Neurochem 76(5):1509–1520
McCully KS (2009) Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endotelial dysfunction, and inflammation. Ann Clin Lab Sci 39(3):219–232
Dammann O, Kuban KCK, Leviton A (2002) Perinatal infection, fetal inflammatory response, white matter damage and cognitive limitation in children born preterm. Ment Retard Dev Disabil Res Rev 8:46–50
Monji A, Kato T, Kanba S (2009) Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 63(3):257–265
Hava G, Vered L, Yael M, Mordechai H, Mahoud H (2006) Alterations in behavior in adultoffspring mice following maternal inflammation during pregnancy. Dev Psychobiol 48(2):162–168
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E (2008) Inflammatory cytokine lterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 63(8):801–808
Golan H, Levav T, Mendelsohn A, Huleihel M (2004) TNF alpha involvement in hippocampus development and function. Cereb Cortex 14:97–105
Barker V, Middleton G, Davey F, Davies A (2001) TNFa contribute to the death of NGF-dependent neurons during development. Nat Neurosci 4:1194–1198
Beattie E, Stellwagen D, Morishita W, Bresnahan J, Ha B, Von Zastrtrow M, Beattie M, Malenka R (2002) Control of synaptic strength by glial TNFa. Science 295:2282–2285
Butler MP, O’Connor JJ, Moynagh PN (2004) Dissection of tumor-necrosis factor-a inhibition of long-term potentiation (LTP) reveals A p38 mitogenactivated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience 124:319–326
Holden G, Bearison DJ, Rode DC, Kapiloff MF, Rosenberg G, Rosenzweig J (2002) The impact of a computer network on pediatric pain and anxiety: a randomized controlled clinical trial. Soc Work Health Care 36(2):21–33
Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H, Wu SX, Xia MZ et al (2013) Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One 8(12):e82713
Parletta N, Milte CM, Meyer BJ (2013) Nutritional modulation of cognitive function and mental health. J Nutr Biochem 24(5):725–743
Mischoulon D, Raab MF (2007) The role of folate in depression and dementia. J Clin Psychiatry 68:28–33
Selhub J, Bagley LC, Miller K, Rosebery IH (2000) B vitamins, homocystein, and neurocognitive function in the elderly. Am J Clin Nutr 71:6145–6205
Tolmunen T, Voutilainen S, Hintikka J, Rissanen T, Tanskanen A, Viinamäki H, Kaplan GA, Salonen JT (2003) Folate and depressive symptoms are associated in middle-aged Finnish men. Am Soc Nutr Sci 133(10):3233–3236
Casper RC (2004) Nutrients, neurodevelopment, and mood. Curr Psychiatry Rep 6(6):425–429
Calvaresi E, Bryan JB (2001) Vitamins, cognition and ageing: a review. J Gerontol B Psychol Sci Soc Sci 56(6):327–339
Hannan-Fletcher MP, Armstrong NC, Scott JM, Pentieva K, Bradbury I, Ward M, Strain JJ, Dunn AA et al (2004) Determining bioavailability of food folate in a controlled intervention study. Am J Clin Nutr 80(4):911–918
Hampson E, Phillips SD, Duff-Canning SJ, Evans KL, Merrill M, Pinsonneault JK, Sadée W, Soares CN et al (2015) Working memory in pregnant women: relation to estrogen and antepartum depression. Horm Behav 74:218–227
Buckwalter JG, Buckwalter DK, Bluestein BW, Stanczyk FZ (2001) Pregnancy and post partum: changes in cognition and mood. Prog Brain Res 133:303–319
Evans J, Heron J, Francomb H, Oke S, Golding J (2001) Cohort study of depressed mood during pregnancy and after childbirth. BMJ 323(7307):257–260
Crawley RA, Dennison K, Carter C (2003) Cognition in pregnancy and the first year post-partum. Psychology Psychotherapy: Psychol Psychother 76(Pt 1):69–84
Prado EL, Ullman MT, Muadz H, Alcock KJ, Shankar AH (2012) The effect of maternal multiple micronutrient supplementation on cognition and mood during pregnancy andpostpartum in Indonesia: a randomized trial. PLoS One 7(3):e32519
Peleg-Raibstein D, Luca E, Wolfrum C (2012) Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res 233(2):398–404
Van Abeelen AF, Elias SG, Bossuyt PM, Grobbee DE, Van der Schouw VT, Roseboom TJ, Uiterwaal CS (2012) Famine exposure in the young and the risk of type 2 diabetes in adulthood. Diabetes 61(9):2255–2260
Zugno AI, Canever L, Heylmann AS, Wessler PG, Steckert A, Mastella GA, de Oliveira MB, Damázio LS et al (2016) Effect of folic acid on oxidative stress and behavioral changes in the animal model of schizophrenia induced by ketamine. J Psychiatr Res 81:23–35
García-Miss Mdel R, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, Gurubel-Maldonado J, Arankowsky-Sandoval G (2010a) Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res 44(7):441–446
Lieberman JA (1999) Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry 46:729–739
Perez-Neri I, Ramirez-Bermudez J, Montes S, Rios C (2006) Possible mechanisms of neurodegeneration in schizophrenia. Neurochem Res 31:1279–1294
Nakki R, Koistinaho J, Sharp FR, Sagar SM (1995) Cerebellar toxicity of phencyclidine. J Neurosci 15:2097–2108
Nakki R, Nickolenko J, Chang J, Sagar SM, Sharp FR (1996) Haloperidol prevents ketamine and phencyclidine-induced HSP70 protein expression but not microglial activation. Exp Neurol 137:234–241
Brocardo PS, Budni J, Lobato KR, Kaster MP, Rodrigues AL (2008a) Antidepressant-like effect of folic acid: involvement of NMDA receptors and L-arginine-nitric oxide-cyclic guanosine monophosphate pathway. Eur J Pharmacol 598(1–3):37–42
Brocardo PS, Budni J, Kaster MP, Santos AR, Rodrigues AL (2008b) Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 54(2):464–473
Brocardo PS, Budni J, Lobato KR, Santos AR, Rodrigues AL (2009) Evidence for the involvement of the opioid system in the antidepressant-like effect of folic acid in the mouse forced swimming test. Behav Brain Res 200(1):122–127
Budni J, Freitas AE, Binfaré RW, Rodrigues AL (2012) Role of potassium channels in the antidepressant-like effect of folic acid in the forced swimming test in mice. Pharmacol Biochem Behav 101(1):148–154
Conrad CD, Galea LA, Kuroda Y, McEwen BS (1996) Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110(6):1321–1334
Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS (1997) The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res 759(1):76–83
Becker A, Grecksch G (2004) Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog Neuro-Psychopharmacol Biol Psychiatry 28(8):1267–1277
Imre G, Fokkema DS, Den Boer JA, Ter Horst GJ (2006) Dose-response characteristics of ketamine effect on locomotion, cognitive function and central neuronal activity. Brain Res Bull 69(3):338–345
Tomiya M, Fukushima T, Kawai J, Aoyama C, Mitsuhashi S, Santa T, Imai K, Toyo'oka T Alterations of plasma and cerebrospinal fluid glutamate levels in rats treated with the N-methyl-D-aspartate receptor antagonist, ketamine. Biomed Chromatogr 20(6–7):628–633
Canever L, Oliveira L, D'altoe De Luca R, Correa PT, De BFD, Matos MP, Scaini G, Quevedo J et al (2010) A rodent model of schizophrenia reveals increase in creatine kinase activity with associated behavior changes. Oxidative Med Cell Longev 3(6):421–427
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Workman JL, Barha CK, Galea LA (2012) Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 126(1):54–72
DeGroot RHM, Vuurman EFPM, Hornstra G, Jolles J (2006) Differences in cognitive performance during pregnancy and early motherhood. Psychol Med 36(7):1023–1032
Clarke R, Refsum H, Birks J, Grimley Evans J, Johnston C, Sherliker P (2003) Screening for vitamin B12 and folate deficiency in older people. Am J Clin Nutr 77(5):1241–1247
Daval JL, Blaise S, Guéant JL (2009) Vitamin B deficiency causes neural cell loss and cognitive impairment in the developing rat. Proc Natl Acad Sci 106(1):E1
Berrocal ZIM, Sequeira MJ, Murphy M, Ballart FDJ, Baki AGS, Bergold JP, Quadros VE (2014) Folate deficiency in rat pups during weaning causes learning and memory déficits. Br J Nutr 112(8):1323–1332
Akchiche N, Bossenmeyer-Pourie C, Kerek R (2012) Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells. FASEB J 26:3980–3992
Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK (2013) Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 25:1039–1061
Singh HJ, Saleh HI, Gupalo S, Omar E (2013) Effect of melatonin supplementation on pregnancy outcome in Wistar-Kyoto and Sprague-Dawley rats. Sheng Li Xue Bao 65(2):149–157
Boldyrev AA, Johnson P (2007) Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal cell glutamate receptors in Alzheimer’s disease. J Alzheimers Dis 11(2):219–228
Roy S, Anvita K, Dangat K, Sable P, Asmita K, Joshi S (2012) Maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids: implications for neurodevelopmental risk in the rat offspring. Brain and Development 34:64–71
Larsson SC, Giovannucci E, Wolk A (2006) Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric and pacreatic cancer: a meta-analysis. Gastroenterology 131(4):1271–1283
D'Souza V, Chavan-Gautam P, Joshi S (2013) Counteracting oxidative stress in pregnancy through modulation of maternal micronutrients and omega-3 fatty acids. Curr Med Chem 20(37):4777–4783
Hague WM (2003) Homocysteine and pregnancy. Best Pract Res Clin Obstet Gynaecol 17(3):459–469
Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho CS, Wajner M, Wyse AT (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Streck EL, Bavaresco CS, Netto CA, Wyse AT (2004) Chronic hyperhomocysteinemia provokes a memory deficit in rats in the Morris water maze task. Behav Brain Res 153:377–381
Henning SM, Swendseid ME, Ivandic BT, Liao F (1997) Vitamins C, E and A and heme oxygenase in rats fed methyl/folate-deficient diets. Free Radic Biol Med 23:936–942
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272(33):20313–20316
Rajendiran S, Swetha Kumari A, Nimesh A, Soundararaghavan S, Ananthanarayanan PH, Dhiman P (2015) Markers of oxidative stress in pregnant women with sleep disturbances. Oman Med J 30(4):264–269
Loscalzo J (1996) The oxidant stress of hyperhomocysteinemia. J Clin Invest 98(1):5–7
Sibrian-Vazquez M, Escobedo JO, Lim S, Samoei GK, Strongin RM (2010) Homocystamides promote free-radical and oxidative damage to proteins. Proc Natl Acad Sci 107(2):551–554
Wang L, Li J, Xie Y, Zhang XG (2010) Association between serum homocysteine and oxidative stress in elderly patients with obstructive sleep apnea/hypopnea syndrome. Biomed Environ Sci 23(1):42–47
McCall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, Zhang CL, Pearce RA et al (1996) Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Nat Acad Sci 93:6361–6366
Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM et al (2010) Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 5(9):e12244
Koz ST, Etem EO, Baydas G, Yuce H, Ozercan HI, Kuloğlu T, Koz S, Etem A et al (2012) Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats. Brain Res 1484:29–38
Mattson MP, Shea TB (2003) Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 26(3):137–146
Xu DX, Wang H, Ning H, Zhao L, Chen YH (2007) Maternally administered mela-tonin differentially regulates lipopolysaccharide-induced proinflammatory andanti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, andfetal brain. J Pineal Res 43:74–79
Feng D, Zhou Y, Xia M, Ma J (2011) Folic acid inhibits lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages by suppressing MAPKs and NF-κB activation. Inflamm Res 60(9):817–822
Hofmann MA, Kohl B, Zumbach MS, Borcea V, Bierhaus A, Henkels M, Amiral J, Schmidt AM et al (1998) Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care 21:841–848
Kamudhamas A, Pang L, Smith SD, Sadovsky Y, Nelson DM (2004) Homocysteine thiolactone induces apoptosis in cultured human trophoblasts: a mechanism for homocysteine-mediated placental dysfunction? Am J Obstet Gynecol 191:563–571
Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P (2001) Signs of asphyxia at birth and risk of schizophrenia: population-based case-control study. Br J Psychiatry 179:403–408
Cannon M, Jones PB, Murray RM (2002) Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 159:1080–1092
Brown AS, Bottiglieri T, Schaefer CA, Quesenberry CP Jr, Liu L, Bresnahan M, Susser ES (2007) Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry 64(1):31–39
Dhobale M (2014) Neurotrophins: role in adverse pregnancy outcome. Int J Dev Neurosci 37:8–14
Müller N, Weidinger E, Leitner B, Schwarz MJ (2015) The role of inflammation in schizophrenia. Front Neurosci. doi:10.3389/fnins.2015.00372
Marques AH, O'Connor TG, Roth C, Susser E, Bjørke-Monsen AL (2013) The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci 7:120
Meyer U, Schwarz MJ, Müller N (2011) Inflammatory processes in schizophrenia: a promising neuroimunological target for the treatment of negative/cognitive symptons and beyond. Pharmacol Ther 132(1):96–110
Na KS, Jung HY, Kim YK (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 48:277–286
Watkins CC, Andrews SR (2016) Clinical studies of neuroinflammatory mechanisms in schizophrenia. Schizophr Res 176(1):14–22
Loix S, De Kock M, Henin P (2011) The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg 62(1):47–58
Cao YL, Zhang W, Ai YQ, Zhang WX, Li Y (2014) Effect of propofol and ketamine anesthesia on cognitive function and immune function in young rats. Asian Pac J Trop Med 7(5):407–411
Feng LG, Song ZW, Xin F, Hu J (2009) Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: a Chinese Han population-based case-control study. Psychiatry Res 168:205–208
García-Miss Mdel R, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, Gurubel-Maldonado J, Arankowsky-Sandoval GJ (2010b) Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphismare risk factors for schizoprenia. Psychiatry Res 44(7):441–446
Amani R (2006) Is dietary pattern of schizophrenia patients different from healthy subjects? Bio Med Central Psychiatry 7:15–19
Friso S, Choi SW (2005) Gene-nutrient interactions in one-carbon metabolism. Curr Drug Metab 6:37–46
Schroecksnadel K, Frick B, Winkler C, Leblhuber F, Wirleitner B, Fuchs D (2003) Hyperhomocysteinemia and immune activation. Clin Chem Lab Med 41:1438–1443
Schroecksnadel K, Frick B, Wirleitner B, Winkler C, Schennach H, Fuchs D (2004) Moderate hyperhomocysteinemia and immune activation. Curr Pharm Biotechnol 5:107–118
Lazzerini PE, Selvi E, Lorenzini S, Capecchi PL, Ghittoni R, Bisogno S, Catenaccio M, Marcolongo R et al (2006) Homocysteine enhances cytokine production in cultured synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol 24:387–393
Zhang L, Jin M, Hu XS, Zhu JH (2006) Homocysteine stimulates nuclear factor kappa B activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int 30:592–597
Wang G, Dai J, Mao J, Zeng X, Yang X, Wang X (2005) Folic acid reverses hyperresponsiveness of LPS-induced chemokine secretion from monocytes in patients with hyperhomocysteinemia. Atherosclerosis 179:395–402
Barua S, Kuizon S, Brown WT, Junaid MA (2016) High gestational folic acid supplementation alters expression of imprinted and candidate autism susceptibility genes in a sex-specific manner in mouse offspring. J Mol Neurosci 58(2):277–286
Acknowledgments
The Laboratory of Neurosciences (Brazil) is one of the centers of the National Institute for Translational Medicine (INCT-TM) and also one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research was supported by grants from CNPq, Instituto Cérebro e Mente, and UNESC. JQ and AIZ are CNPq fellows.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animals were used according to the NIH Guide for the Care and Use of Laboratory Animals. The local Ethical Committee for Animal Research (CEUA 100-2014-01/UNESC) approved the protocol and all experiments. All efforts were made to minimize animal suffering and to reduce the number of animals used in the experiment.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper. Zugno and Budni had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Rights and permissions
About this article
Cite this article
Canever, L., Alves, C.S.V., Mastella, G. et al. The Evaluation of Folic Acid-Deficient or Folic Acid-Supplemented Diet in the Gestational Phase of Female Rats and in Their Adult Offspring Subjected to an Animal Model of Schizophrenia. Mol Neurobiol 55, 2301–2319 (2018). https://doi.org/10.1007/s12035-017-0493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0493-7