Abstract
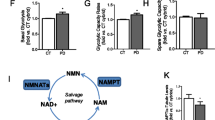
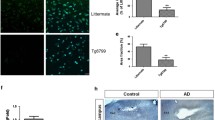
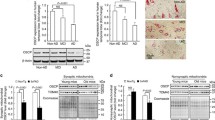
Multiple lines of evidence state a major role for mitochondrial dysfunction in sporadic Alzheimer’s disease (AD) etiopathogenesis. However, the molecular mechanism(s) triggered by mitochondrial deficits that lead to neurodegeneration remain elusive. Herein, we propose a new mechanism by which mitochondrial loss of potential leads to a dysfunction in autophagy/mitophagy due to the overactivation of SIRT2, a tubulin deacetylase that regulates microtubule network acetylation, and provide insights into the association between metabolism, phosphorylation, and Aβ aggregation. We observed an increase in SIRT2 levels and a decrease in the acetylation of lys40 of tubulin in AD cells containing patient mtDNA as well as in AD brains. SIRT2 loss of function either with AK1 (a specific SIRT2 inhibitor) or by SIRT2 knockout recovers microtubule stabilization and improves autophagy, favoring cell survival through the elimination of toxic Aβ oligomers. Our data provide strong evidence for a functional role of tubulin acetylation on autophagic vesicle traffic and mitochondria degradation. We propose that SIRT2 inhibition may improve microtubule assembly thus representing a valid approach as disease-modifying therapy for AD.








Similar content being viewed by others
References
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 1842(8):1219–1231. doi:10.1016/j.bbadis.2013.09.010
Parker WD Jr, Filley CM, Parks JK (1990) Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 40(8):1302–1303
Swerdlow RH, Khan SM (2004) A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses 63(1):8–20. doi:10.1016/j.mehy.2003.12.045
Silva DF, Selfridge JE, Lu J, Roy ELN, Hutfles L, Burns JM, Michaelis EK, Yan S et al (2013) Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet 22(19):3931–3946. doi:10.1093/hmg/ddt247
Silva DF, Santana I, Esteves AR, Baldeiras I, Arduino DM, Oliveira CR, Cardoso SM (2013) Prodromal metabolic phenotype in MCI cybrids: implications for Alzheimer’s disease. Curr Alzheimer Res 10(2):180–190
Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L et al (2006) Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion 6(6):323–330. doi:10.1016/j.mito.2006.10.004
Satoh A, Imai S (2014) Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat Commun 5:4211. doi:10.1038/ncomms5211
Harting K, Knoll B (2010) SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol 89(2–3):262–269. doi:10.1016/j.ejcb.2009.11.006
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD + −dependent tubulin deacetylase. Mol Cell 11(2):437–444
Stokin GB, Goldstein LS (2006) Axonal transport and Alzheimer’s disease. Annu Rev Biochem 75:607–627. doi:10.1146/annurev.biochem.75.103004.142637
Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE (1995) The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem 270(34):19791–19799
Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV et al (2003) Microtubule reduction in Alzheimer’s disease and aging is independent of filament formation. Am J Pathol 162(5):1623–1627
Hempen B, Brion JP (1996) Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J Neuropathol Exp Neurol 55(9):964–972
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y et al (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21(24):6820–6831
Silva DF, Esteves AR, Oliveira CR, Cardoso SM (2011) Mitochondria: the common upstream driver of amyloid-beta and pathology in Alzheimer’s disease. Curr Alzheimer Res 8(5):563–572
Cardoso SM, Santana I, Swerdlow RH, Oliveira CR (2004) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem 89(6):1417–1426. doi:10.1111/j.1471-4159.2004.02438.x
Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP Jr, Miller SW, Davis RE, Parker WD Jr (2000) Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol 162(1):37–50. doi:10.1006/exnr.2000.7333
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13(1):72–78. doi:10.1002/ana.410130116
Saraiva AA, Borges MM, Madeira MD, Tavares MA, Paula-Barbosa MM (1985) Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer’s disease. J Submicrosc Cytol 17(3):459–464
Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45(8):836–840
Zhu X, Perry G, Smith MA, Wang X (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimer’s Dis : JAD 33(Suppl 1):S253–S262. doi:10.3233/JAD-2012-129005
Terry RD (1998) The cytoskeleton in Alzheimer disease. J Neural Transm Suppl 53:141–145
Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA et al (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci : Off J Soc Neurosci 27(10):2606–2616. doi:10.1523/JNEUROSCI.4181-06.2007
Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E et al (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180(5):915–929. doi:10.1083/jcb.200707126
Suzuki K, Koike T (2007) Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience 147(3):599–612. doi:10.1016/j.neuroscience.2007.04.059
Encalada SE, Goldstein LS (2014) Biophysical challenges to axonal transport: motor-cargo deficiencies and neurodegeneration. Annu Rev Biophys 43:141–169. doi:10.1146/annurev-biophys-051013-022746
Trimmer PA, Borland MK (2005) Differentiated Alzheimer’s disease transmitochondrial cybrid cell lines exhibit reduced organelle movement. Antioxid Redox Signal 7(9–10):1101–1109. doi:10.1089/ars.2005.7.1101
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci : Off J Soc Neurosci 28(27):6926–6937. doi:10.1523/JNEUROSCI.0800-08.2008
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M (2013) Impaired autophagy and APP processing in Alzheimer’s disease: the potential role of Beclin 1 interactome. Prog Neurobiol 106–107:33–54. doi:10.1016/j.pneurobio.2013.06.002
Butler D, Hwang J, Estick C, Nishiyama A, Kumar SS, Baveghems C, Young-Oxendine HB, Wisniewski ML et al (2011) Protective effects of positive lysosomal modulation in Alzheimer’s disease transgenic mouse models. PLoS One 6(6):e20501. doi:10.1371/journal.pone.0020501
Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, Gonzales E, Burchett JM et al (2014) Enhancing astrocytic lysosome biogenesis facilitates Abeta clearance and attenuates amyloid plaque pathogenesis. J Neurosci : Off J Soc Neurosci 34(29):9607–9620. doi:10.1523/JNEUROSCI.3788-13.2014
Salminen A, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H, Alafuzoff I (2012) Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 96(1):87–95. doi:10.1016/j.pneurobio.2011.11.005
Okatsu K, Oka T, Iguchi M, Imamura K, Kosako H, Tani N, Kimura M, Go E et al (2012) PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun 3:1016. doi:10.1038/ncomms2016
Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK (2011) The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117(5):856–867. doi:10.1111/j.1471-4159.2011.07253.x
Perry G, Mulvihill P, Fried VA, Smith HT, Grundke-Iqbal I, Iqbal K (1989) Immunochemical properties of ubiquitin conjugates in the paired helical filaments of Alzheimer disease. J Neurochem 52(5):1523–1528
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120(Pt 23):4081–4091. doi:10.1242/jcs.019265
Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C et al (2008) A block of autophagy in lysosomal storage disorders. Hum Mol Genet 17(1):119–129. doi:10.1093/hmg/ddm289
Lee S, Sato Y, Nixon RA (2011) Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy 7(12):1562–1563
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM (2008) Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phospho. J Neurosci : Off J Soc Neurosci 28(45):11500–11510. doi:10.1523/JNEUROSCI.3203-08.2008
Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I (2009) Mechanisms of -induced neurodegeneration. Acta Neuropathol 118(1):53–69. doi:10.1007/s00401-009-0486-3
Cai Z, Zhou Y, Liu Z, Ke Z, Zhao B (2015) Autophagy dysfunction upregulates beta-amyloid peptides via enhancing the activity of gamma-secretase complex. Neuropsychiatr Dis Treat 11:2091–2099. doi:10.2147/NDT.S84755
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M et al (2005) Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171(1):87–98. doi:10.1083/jcb.200505082
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Zhang F, Su B, Wang C, Siedlak SL, Mondragon-Rodriguez S, Lee HG, Wang X, Perry G et al (2015) Posttranslational modifications of alpha-tubulin in Alzheimer disease. Translat Neurodegenera 4:9. doi:10.1186/s40035-015-0030-4
Reddy PH (2014) Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimer’s Dis : JAD 40(2):245–256. doi:10.3233/JAD-132060
Gan X, Huang S, Wu L, Wang Y, Hu G, Li G, Zhang H, Yu H et al (2014) Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta 1842(2):220–231. doi:10.1016/j.bbadis.2013.11.009
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neruomol Med 5(2):147–162. doi:10.1385/NMM:5:2:147
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci : Off J Soc Neurosci 29(28):9090–9103. doi:10.1523/JNEUROSCI.1357-09.2009
Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173(2):470–482. doi:10.2353/ajpath.2008.071208
Esteves AR, Santos MGFD, Januario C, Cardoso SM (2015) The Upshot of LRRK2 Inhibition to Parkinson’s disease paradigm. Mol Neurobiol 52(3):1804–1820. doi:10.1007/s12035-014-8980-6
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D et al (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain : J Neurol 134(Pt 1):258–277. doi:10.1093/brain/awq341
Nilsson P, Saido TC (2014) Dual roles for autophagy: degradation and secretion of Alzheimer’s disease Abeta peptide. BioEssays : News Rev Molec, Cell Dev Biol 36(6):570–578. doi:10.1002/bies.201400002
Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S, Tanaka M, Iwata N et al (2013) Abeta secretion and plaque formation depend on autophagy. Cell Rep 5(1):61–69. doi:10.1016/j.celrep.2013.08.042
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B et al (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 118(6):2190–2199. doi:10.1172/JCI33585
Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T (2010) Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One 5(6):e11102. doi:10.1371/journal.pone.0011102
Goldstein LS (2012) Axonal transport and neurodegenerative disease: can we see the elephant? Prog Neurobiol 99(3):186–190. doi:10.1016/j.pneurobio.2012.03.006
Millecamps S, Julien JP (2013) Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 14(3):161–176. doi:10.1038/nrn3380
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64(2):113–122
Sanchez-Varo R, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, De Castro V, Jimenez S et al (2012) Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol 123(1):53–70. doi:10.1007/s00401-011-0896-x
Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM (2011) Amyloid-beta-induced mitochondrial dysfunction impairs the autophagic lysosomal pathway in a tubulin dependent pathway. J Alzheimer’s Dis : JAD 26(3):565–581. doi:10.3233/JAD-2011-110423
Miyasaka T, Sato S, Tatebayashi Y, Takashima A (2010) Microtubule destruction induces liberation and its subsequent phosphorylation. FEBS Lett 584(14):3227–3232. doi:10.1016/j.febslet.2010.06.014
Mondragon-Rodriguez S, Perry G, Luna-Munoz J, Acevedo-Aquino MC, Williams S (2014) Phosphorylation of protein at sites Ser(396–404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol Appl Neurobiol 40(2):121–135. doi:10.1111/nan.12084
Ding H, Matthews TA, Johnson GV (2006) Site-specific phosphorylation and caspase cleavage differentially impact -microtubule interactions and aggregation. J Biol Chem 281(28):19107–19114. doi:10.1074/jbc.M511697200
Xie R, Nguyen S, McKeehan WL, Liu L (2010) Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 11:89. doi:10.1186/1471-2121-11-89
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol : CB 16(21):2166–2172. doi:10.1016/j.cub.2006.09.014
Bendiske J, Bahr BA (2003) Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis—an approach for slowing Alzheimer disease? J Neuropathol Exp Neurol 62(5):451–463
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM et al (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. doi:10.1126/science.1143780
Hasegawa T, Baba T, Kobayashi M, Konno M, Sugeno N, Kikuchi A, Itoyama Y, Takeda A (2010) Role of TPPP/p25 on alpha-synuclein-mediated oligodendroglial degeneration and the protective effect of SIRT2 inhibition in a cellular model of multiple system atrophy. Neurochem Int 57(8):857–866. doi:10.1016/j.neuint.2010.09.002
Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL et al (2010) SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A 107(17):7927–7932. doi:10.1073/pnas.1002924107
Gal J, Bang Y, Choi HJ (2012) SIRT2 interferes with autophagy-mediated degradation of protein aggregates in neuronal cells under proteasome inhibition. Neurochem Int 61(7):992–1000. doi:10.1016/j.neuint.2012.07.010
Spires-Jones TL, Fox LM, Rozkalne A, Pitstick R, Carlson GA, Kazantsev AG (2012) Inhibition of Sirtuin 2 with sulfobenzoic acid derivative AK1 is non-toxic and potentially neuroprotective in a mouse model of frontotemporal dementia. Front Pharmacol 3:42. doi:10.3389/fphar.2012.00042
Miller SW, Trimmer PA, Parker WD Jr, Davis RE (1996) Creation and characterization of mitochondrial DNA-depleted cell lines with “neuronal-like” properties. J Neurochem 67(5):1897–1907
Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP Jr, Davis RE et al (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol 40(4):663–671. doi:10.1002/ana.410400417
Serrano L, Martinez-Redondo P, Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB, Kane-Goldsmith N et al (2013) The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev 27(6):639–653. doi:10.1101/gad.211342.112
Agostinho P, Oliveira CR (2003) Involvement of calcineurin in the neurotoxic effects induced by amyloid-beta and prion peptides. Eur J Neurosci 17(6):1189–1196
Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, Budka H, Carmona M et al (2007) Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol 66(1):35–46. doi:10.1097/nen.0b013e31802c3e7d
Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 284(20):13843–13855. doi:10.1074/jbc.M808515200
Cregan SP, MacLaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS (1999) Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci : Off J Soc Neurosci 19(18):7860–7869
Acknowledgments
We are grateful to Dr. Russell H Swerdlow for providing the AD patient samples. Human brain samples were obtained from the Neurological Tissue Bank, Biobanc-Hospital Clinic-IDIBAPS and from a generous gift from Professor I Ferrer Abizanda, Bellvitge Hospital Universitari, Institut Català de la Salut, Barcelona, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by funds from PEst-C/SAU/LA0001/2011-2013 to SM Cardoso. AR Esteves and DF Silva are supported by postdoctoral fellowships from the Portuguese Foundation for Science and Technology (FCT-MCTES, Portugal).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Silva, D.F., Esteves, A.R., Oliveira, C.R. et al. Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer’s-Disease Related Pathology. Mol Neurobiol 54, 4021–4040 (2017). https://doi.org/10.1007/s12035-016-9951-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9951-x