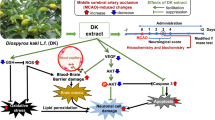
Abstract
Oxidative stress and inflammatory responses play a critical contributing factor in cerebral ischemia and reperfusion, which lead to lipid peroxidation and neuronal dysfunction that may represent a target for therapeutic intervention. The present study was aimed to elucidate the neuroprotective effect of tannic acid (TA), a natural polyphenol with potential antioxidant and antiinflammatory properties on middle cerebral artery occlusion (MCAO) model in rats. To test this hypothesis, male Wistar rats were pretreated with TA (50 mg/kg b.wt.) and then subjected to 2-h MCAO followed by 22 h of reperfusion. After 2-h MCAO/22-h reperfusion, neurological deficit, infarct sizes, activities of antioxidant enzymes, cytokine level, histology, and immunohistochemistry were used to analyze the expression of glial fibrillary acidic protein (GFAP) in ischemic brain. The pretreatment of TA showed a marked reduction in infarct size, improved neurological function, suppressed neuronal loss, and downregulated the GFAP expression in MCAO rats. A significantly depleted activity of antioxidant enzymes and content of glutathione in MCAO group were protected significantly in MCAO group pretreated with TA. Conversely, the elevated level of thiobarbituric acid reactive species and cytokines in MCAO group was attenuated significantly in TA-pretreated group when compared with MCAO group. The results indicated that TA protected the brain from damage caused by MCAO, and this effect may thorough diminish the oxidative stress and inflammatory responses.







Similar content being viewed by others
References
Schimidt HL, Vieira A, Altermann C, Martins A, Sosa P, Santos FW, Mello-Carpes PB, Izquierdo I, et al. (2014) Memory deficits and oxidative stress in cerebral ischemia-reperfusion: neuroprotective role of physical exercise and green tea supplementation. Neurobiol Learn Mem 114:242–250
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87:179–197
Chen H, Hong H, Liu D, Xu G, Wang Y, Zeng J, Zhang R, Liu X (2011) Lesion patterns and mechanism of cerebral infarction caused by severe atherosclerotic intracranial internal carotid artery stenosis. J Neurol Sci 307:79–85
Sun J, Li YZ, Ding YH, Wang J, Geng J, Yang H, Ren J, Tang JY, et al. (2014) Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res 1589:126–139
Halladin NL (2015) Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan Med J 62:B5054
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97
Hennessy E, Griffin EW, Cunningham C (2015) Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1beta and TNF-alpha. J Neurosci 35:8411–8422
Ahmad A, Khan MM, Javed H, Raza SS, Ishrat T, Khan MB, Safhi MM, Islam F (2012) Edaravone ameliorates oxidative stress associated cholinergic dysfunction and limits apoptotic response following focal cerebral ischemia in rat. Mol Cell Biochem 367:215–225
Fang L, Gao H, Zhang W, Wang Y (2015) Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int J Clin Exp Med 8:3219–3226
Krajka-Kuzniak V, Kaczmarek J, Baer-Dubowska W (2008) Effect of naturally occurring phenolic acids on the expression of glutathione S-transferase isozymes in the rat. Food Chem Toxicol 46:1097–1102
Winiarska-Mieczan A (2013) Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ Toxicol Pharmacol 36:9–18
Tikoo K, Bhatt DK, Gaikwad AB, Sharma V, Kabra DG (2007) Differential effects of tannic acid on cisplatin induced nephrotoxicity in rats. FEBS Lett 581:2027–2035
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, Javed H, Siddiqui MS, et al. (2011) Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res 1420:93–105
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Zaheer N, Tewari KK, Krishnan PS (1965) Exposure and solubilization of hepatic mitochondrial shunt dehydrogenases. Arch Biochem Biophys 109:646–648
Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA (2000) Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006–1015
Claiborne A (1985) Catalase activity. In: Green Wald RA (ed) CRC hand book of methods for oxygen radical research. CRC Press, Boca Raton, FL, pp. 283–284
Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG (1993) A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214:11–16
Khan MM, Raza SS, Javed H, Ahmad A, Khan A, Islam F, Safhi MM (2012) Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res 22:1–15
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Yuan Y, Zha H, Rangarajan P, Ling EA, Wu C (2014) Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia. BMC Neurosci 15:125
Palencia G, Medrano JA, Ortiz-Plata A, Farfan DJ, Sotelo J, Sanchez A, Trejo-Solis C (2015) Anti-apoptotic, anti-oxidant, and anti-inflammatory effects of thalidomide on cerebral ischemia/reperfusion injury in rats. J Neurol Sci 351:78–87
Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ahmed ME, Ashafaq M, Khan MB, Islam F, et al. (2015) Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-kappaB in middle cerebral artery occlusion rats. Eur J Pharmacol 747:190–199
O’Keeffe F, Liegeois F, Eve M, Ganesan V, King J, Murphy T (2014) Neuropsychological and neurobehavioral outcome following childhood arterial ischemic stroke: attention deficits, emotional dysregulation, and executive dysfunction. Child Neuropsychol 20:557–582
Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C (2013) Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology 71:154–163
Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S (2010) The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab 30:1951–1961
Rakhunde PB, Saher S, Ali SA (2014) Neuroprotective effect of Feronia limonia on ischemia reperfusion induced brain injury in rats. Indian J Pharmacol 46:617–621
Tuzmen MN, Yucel NC, Kalburcu T, Demiryas N (2015) Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol Mech Methods 25:120–127
Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB, Raza SS, Javed H, et al. (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292:123–135
Shi H, Jing X, Wei X, Perez RG, Ren M, Zhang X, Lou H (2015) S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J Neurochem 133:298–308
Lee JC, Won MH (2014) Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat Cell Biol 47:149–156
Atif F, Yousuf S, Agrawal SK (2009) S-allyl L-cysteine diminishes cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. Brain Res 1265:128–137
Nabavi SF, Dean OM, Turner A, Sureda A, Daglia M, Nabavi SM (2015) Oxidative stress and post-stroke depression: possible therapeutic role of polyphenols? Curr Med Chem 22:343–351
Yunoki T, Deguchi K, Omote Y, Liu N, Liu W, Hishikawa N, Yamashita T, Abe K (2014) Anti-oxidative nutrient-rich diet protects against acute ischemic brain damage in rats. Brain Res 1587:33–39
Dziedzic T (2015) Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother 15:523–531
Sen HM, Ozkan A, Guven M, Akman T, Aras AB, Sehitoglu I, Alacam H, Silan C, et al. (2015) Effects of tannic acid on the ischemic brain tissue of rats. Inflammation 38:1624–1630
Ahmad A, Khan MM, Raza SS, Javed H, Ashafaq M, Islam F, Safhi MM (2012) Ocimum sanctum attenuates oxidative damage and neurological deficits following focal cerebral ischemia/reperfusion injury in rats. Neurol Sci 33:1239–1247
Yao Y, Chen L, Xiao J, Wang C, Jiang W, Zhang R, Hao J (2014) Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int J Mol Sci 15:20913–20926
Acknowledgments
Dr. Mohammad Ashafaq was supported by a Research Associateship from the Indian Council of Medical Research (No. 45/82/2011 PHA-BMS), Government of India. Dr. Heena Tabassum is grateful to Department of Biotechnology, Government of India, for financial grant (DBT BioCARe Program, sanction no. BT/Bio-CARe/01/10219/2013-14). The Grant (no. F. 30-1/2013(SA-II)/RA-2012-14-GE-WES-2400), received as Research Award (2012–14) from the University Grants Commission (UGC), New Delhi, Government of India, to Dr. Suhel Parvez, is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Mohammad Ashafaq and Heena Tabassum contributed equally to the study.
Rights and permissions
About this article
Cite this article
Ashafaq, M., Tabassum, H. & Parvez, S. Modulation of Behavioral Deficits and Neurodegeneration by Tannic Acid in Experimental Stroke Challenged Wistar Rats. Mol Neurobiol 54, 5941–5951 (2017). https://doi.org/10.1007/s12035-016-0096-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0096-8