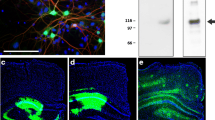
Abstract
SIRT1 induces cell survival and has shown neuroprotection against amyloid and tau pathologies in Alzheimer’s disease (AD). However, protective effects against memory loss or the enhancement of cognitive functions have not yet been proven. We aimed to investigate the benefits induced by SIRT1 overexpression in the hippocampus of the AD mouse model 3xTg-AD and in control non-transgenic mice. A lentiviral vector encoding mouse SIRT1 or GFP, selectively transducing neurons, was injected into the dorsal CA1 hippocampal area of 4-month-old mice. Six-month overexpression of SIRT1 fully preserved learning and memory in 10-month-old 3xTg-AD mice. Remarkably, SIRT1 also induced cognitive enhancement in healthy non-transgenic mice. Neuron cultures of 3xTg-AD mice, which show traits of AD-like pathology, and neuron cultures from non-transgenic mice were also transduced with lentiviral vectors to analyze beneficial SIRT1 mechanisms. We uncovered novel pathways of SIRT1 neuroprotection through enhancement of cell proteostatic mechanisms and activation of neurotrophic factors not previously reported such as GDNF, present in both AD-like and healthy neurons. Therefore, SIRT1 may increase neuron function and resilience against AD.







Similar content being viewed by others
References
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. doi:10.1016/j.cell.2013.05.039
Alzheimer’s Disease International (2015) World Alzheimer Report 2015. Alzheimer’s Disease International (ADI) London
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. doi:10.1126/science.1072994
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120(4):545–555. doi:10.1016/j.cell.2005.02.008
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766
Parsons CG, Danysz W, Dekundy A, Pulte I (2013) Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res 24(3):358–369. doi:10.1007/s12640-013-9398-z
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295. doi:10.1146/annurev.pathol.4.110807.092250
Bosch-Presegue L, Vaquero A (2015) Sirtuin-dependent epigenetic regulation in the maintenance of genome integrity. FEBS J 282(9):1745–1767. doi:10.1111/febs.13053
Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81(3):471–483. doi:10.1016/j.neuron.2014.01.028
Paraiso AF, Mendes KL, Santos SH (2013) Brain activation of SIRT1: role in neuropathology. Mol Neurobiol 48(3):681–689. doi:10.1007/s12035-013-8459-x
Donmez G (2012) The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33(9):494–501. doi:10.1016/j.tips.2012.05.007
Saftig P, Lichtenthaler SF (2015) The alpha secretase ADAM10: a metalloprotease with multiple functions in the brain. Prog Neurobiol 135:1–20. doi:10.1016/j.pneurobio.2015.10.003
Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD (2014) Cilostazol suppresses beta-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-beta. J Neurosci Res 92(11):1581–1590. doi:10.1002/jnr.23421
Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67(6):953–966. doi:10.1016/j.neuron.2010.08.044
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85. doi:10.1186/1750-1326-6-85
Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30(29):9695–9707. doi:10.1523/JNEUROSCI.0027-10.2010
Julien C, Tremblay C, Emond V, Lebbadi M, Salem N Jr, Bennett DA, Calon F (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 68(1):48–58. doi:10.1097/NEN.0b013e3181922348
Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG (2014) Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. Neruomol Med 16(2):405–414. doi:10.1007/s12017-014-8288-8
Marques SC, Lemos R, Ferreiro E, Martins M, de Mendonca A, Santana I, Outeiro TF, Pereira CM (2012) Epigenetic regulation of BACE1 in Alzheimer’s disease patients and in transgenic mice. Neuroscience 220:256–266. doi:10.1016/j.neuroscience.2012.06.029
Torres-Lista V, Parrado-Fernandez C, Alvarez-Monton I, Frontinan-Rubio J, Duran-Prado M, Peinado JR, Johansson B, Alcain FJ, Gimenez-Llort L (2014) Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice. Behav Brain Res 271:140–146. doi:10.1016/j.bbr.2014.04.055
Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R (2014) Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 81:55–63. doi:10.1016/j.neuropharm.2014.01.037
Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, LaFerla FM, Kitazawa M (2014) Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis 42(4):1229–1238. doi:10.3233/JAD-140204
Quintas A, de Solis AJ, Diez-Guerra FJ, Carrascosa JM, Bogonez E (2012) Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol 47(2):198–201. doi:10.1016/j.exger.2011.11.010
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26(13):3169–3179. doi:10.1038/sj.emboj.7601758
Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM (2008) Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 1147:335–347. doi:10.1196/annals.1427.024
Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M (2010) Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun 1:3. doi:10.1038/ncomms1001
Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18(3):416–430. doi:10.1016/j.cmet.2013.07.013
Vilchez D, Saez I, Dillin A (2014) The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5:5659. doi:10.1038/ncomms6659
Tomita T, Hamazaki J, Hirayama S, McBurney MW, Yashiroda H, Murata S (2015) Sirt1-deficiency causes defective protein quality control. Sci Rep 5:12613. doi:10.1038/srep12613
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47:e147. doi:10.1038/emm.2014.117
Himeno E, Ohyagi Y, Ma L, Nakamura N, Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T, Tabira T, LaFerla FM, Kira J (2011) Apomorphine treatment in Alzheimer mice promoting amyloid-beta degradation. Ann Neurol 69(2):248–256. doi:10.1002/ana.22319
Wang DS, Dickson DW, Malter JS (2006) Beta-amyloid degradation and Alzheimer’s disease. J Biomed Biotechnol 2006(3):58406. doi:10.1155/JBB/2006/58406
Godoy JA, Zolezzi JM, Braidy N, Inestrosa NC (2014) Role of Sirt1 during the ageing process: relevance to protection of synapses in the brain. Mol Neurobiol 50(3):744–756. doi:10.1007/s12035-014-8645-5
Codocedo JF, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC (2012) SIRT1 regulates dendritic development in hippocampal neurons. PLoS One 7(10):e47073. doi:10.1371/journal.pone.0047073
Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J (2011) Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res 89(11):1723–1736. doi:10.1002/jnr.22725
Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR 3rd, Bordone L, Guarente L, Krainc D (2012) Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med 18(1):159–165. doi:10.1038/nm.2559
Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466(7310):1105–1109. doi:10.1038/nature09271
Pertusa M, Garcia-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, Cristofol R, Sarkis C, Sanfeliu C (2008) Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging 29(9):1366–1379. doi:10.1016/j.neurobiolaging.2007.02.026
Kutner RH, Zhang XY, Reiser J (2009) Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 4(4):495–505. doi:10.1038/nprot.2009.22
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Garcia-Mesa Y, Colie S, Corpas R, Cristofol R, Comellas F, Nebreda AR, Gimenez-Llort L, Sanfeliu C (2015) Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glv005
Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, Revilla S, Guerra R, Gruart A, Laferla FM, Cristofol R, Delgado-Garcia JM, Sanfeliu C (2011) Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis 24(3):421–454. doi:10.3233/JAD-2011-101635
Caroni P (1997) Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 71(1):3–9
Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM (2005) Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J Neurochem 94(6):1711–1718. doi:10.1111/j.1471-4159.2005.03332.x
Frazzini V, Guarnieri S, Bomba M, Navarra R, Morabito C, Mariggio MA, Sensi SL (2016) Altered Kv2.1 functioning promotes increased excitability in hippocampal neurons of an Alzheimer’s disease mouse model. Cell Death Dis 7:e2100. doi:10.1038/cddis.2016.18
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106(34):14670–14675. doi:10.1073/pnas.0903563106
Sensi SL, Rapposelli IG, Frazzini V, Mascetra N (2008) Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease. Exp Gerontol 43(5):488–492. doi:10.1016/j.exger.2007.10.018
Vale C, Alonso E, Rubiolo JA, Vieytes MR, LaFerla FM, Gimenez-Llort L, Botana LM (2010) Profile for amyloid-beta and tau expression in primary cortical cultures from 3xTg-AD mice. Cell Mol Neurobiol 30(4):577–590. doi:10.1007/s10571-009-9482-3
Alonso E, Vale C, Vieytes MR, Botana LM (2013) Translocation of PKC by yessotoxin in an in vitro model of Alzheimer’s disease with improvement of tau and beta-amyloid pathology. ACS Chem Neurosci 4(7):1062–1070. doi:10.1021/cn400018y
Alonso E, Vale C, Vieytes MR, Laferla FM, Gimenez-Llort L, Botana LM (2011) 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Abeta and hyperphosphorylated tau in vitro. Neurochem Int 59(7):1056–1065. doi:10.1016/j.neuint.2011.08.013
Alonso E, Vale C, Vieytes MR, Laferla FM, Gimenez-Llort L, Botana LM (2011) The cholinergic antagonist gymnodimine improves Abeta and tau neuropathology in an in vitro model of Alzheimer disease. Cell Physiol Biochem 27(6):783–794. doi:10.1159/000330086
Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, Tobena A, LaFerla FM, Fernandez-Teruel A (2007) Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev 31(1):125–147. doi:10.1016/j.neubiorev.2006.07.007
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36(1):60–90
Slevin M, Matou S, Zeinolabediny Y, Corpas R, Weston R, Liu D, Boras E, Di Napoli M, Petcu E, Sarroca S, Popa-Wagner A, Love S, Font MA, Potempa LA, Al-Baradie R, Sanfeliu C, Revilla S, Badimon L, Krupinski J (2015) Monomeric C-reactive protein—a key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Sci Rep 5:13281. doi:10.1038/srep13281
Broadbent NJ, Gaskin S, Squire LR, Clark RE (2010) Object recognition memory and the rodent hippocampus. Learn Mem 17(1):5–11. doi:10.1101/lm.1650110
Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA (2008) SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135(5):907–918. doi:10.1016/j.cell.2008.10.025
Agostinho P, Pliassova A, Oliveira CR, Cunha RA (2015) Localization and trafficking of amyloid-beta protein precursor and secretases: impact on Alzheimer’s disease. J Alzheimers Dis 45(2):329–347. doi:10.3233/JAD-142730
Epis R, Marcello E, Gardoni F, Vastagh C, Malinverno M, Balducci C, Colombo A, Borroni B, Vara H, Dell’Agli M, Cattabeni F, Giustetto M, Borsello T, Forloni G, Padovani A, Di Luca M (2010) Blocking ADAM10 synaptic trafficking generates a model of sporadic Alzheimer’s disease. Brain 133(11):3323–3335. doi:10.1093/brain/awq217
Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 100(7):4162–4167. doi:10.1073/pnas.0230450100
Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D (2012) 5-Lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of Alzheimer disease. Ann Neurol 72(3):442–454. doi:10.1002/ana.23642
Stargardt A, Gillis J, Kamphuis W, Wiemhoefer A, Kooijman L, Raspe M, Benckhuijsen W, Drijfhout JW, Hol EM, Reits E (2013) Reduced amyloid-beta degradation in early Alzheimer’s disease but not in the APPswePS1dE9 and 3xTg-AD mouse models. Aging Cell 12(3):499–507. doi:10.1111/acel.12074
Lloret A, Fuchsberger T, Giraldo E, Vina J (2015) Molecular mechanisms linking amyloid beta toxicity and tau hyperphosphorylation in Alzheimers disease. Free Radic Biol Med 83:186–191. doi:10.1016/j.freeradbiomed.2015.02.028
Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM, Trojanowski JQ (2012) Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain 135(Pt 3):807–818. doi:10.1093/brain/aws013
Morris M, Knudsen GM, Maeda S, Trinidad JC, Ioanoviciu A, Burlingame AL, Mucke L (2015) Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci 18(8):1183–1189. doi:10.1038/nn.4067
Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, Gan L (2015) Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21(10):1154–1162. doi:10.1038/nm.3951
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM (2008) Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci 28(45):11500–11510. doi:10.1523/JNEUROSCI.3203-08.2008
Westerheide SD, Anckar J, Stevens SM Jr, Sistonen L, Morimoto RI (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323(5917):1063–1066. doi:10.1126/science.1165946
Magrane J, Smith RC, Walsh K, Querfurth HW (2004) Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci 24(7):1700–1706. doi:10.1523/JNEUROSCI.4330-03.2004
Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13(7):703–714. doi:10.1093/hmg/ddh083
Keller JN, Hanni KB, Markesbery WR (2000) Impaired proteasome function in Alzheimer’s disease. J Neurochem 75(1):436–439
Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM (2008) Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging 29(11):1607–1618. doi:10.1016/j.neurobiolaging.2007.04.014
Sampaio-Marques B, Ludovico P (2015) Sirtuins and proteolytic systems: implications for pathogenesis of synucleinopathies. Biomolecules 5(2):735–757. doi:10.3390/biom5020735
Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1. doi:10.3389/neuro.02.001.2010
Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M (1997) Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res 49(1–2):71–81
Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15(3):331–337. doi:10.1038/nm.1912
d’Anglemont de Tassigny X, Pascual A, Lopez-Barneo J (2015) GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front Neuroanat 9:10. doi:10.3389/fnana.2015.00010
Revilla S, Ursulet S, Alvarez-Lopez MJ, Castro-Freire M, Perpina U, Garcia-Mesa Y, Bortolozzi A, Gimenez-Llort L, Kaliman P, Cristofol R, Sarkis C, Sanfeliu C (2014) Lenti-GDNF gene therapy protects against Alzheimer’s disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci Ther 20(11):961–972. doi:10.1111/cns.12312
Religa P, Cao R, Religa D, Xue Y, Bogdanovic N, Westaway D, Marti HH, Winblad B, Cao Y (2013) VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci Rep 3:2053. doi:10.1038/srep02053
Silvennoinen M, Ahtiainen JP, Hulmi JJ, Pekkala S, Taipale RS, Nindl BC, Laine T, Hakkinen K, Selanne H, Kyrolainen H, Kainulainen H (2015) PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep 3(10). doi:10.14814/phy2.12563
Reed SM, Quelle DE (2014) p53 acetylation: regulation and consequences. Cancers (Basel) 7(1):30–69. doi:10.3390/cancers7010030
Cai Y, Xu L, Xu H, Fan X (2016) SIRT1 and neural cell fate determination. Mol Neurobiol 53(5):2815–2825. doi:10.1007/s12035-015-9158-6
Lima LC, Saliba SW, Andrade JM, Cunha ML, Cassini-Vieira P, Feltenberger JD, Barcelos LS, Guimaraes AL, de-Paula AM, de Oliveira AC, Santos SH (2016) Neurodegeneration alters metabolic profile and Sirt 1 signaling in high-fat-induced obese mice. Mol Neurobiol. doi:10.1007/s12035-016-9927-x
Michan S (2014) Calorie restriction and NAD(+)/sirtuin counteract the hallmarks of aging. Front Biosci (Landmark Ed) 19:1300–1319
Suwa M, Sakuma K (2013) The potential role of sirtuins regarding the effects of exercise on aging- related diseases. Curr Aging Sci 6(2):178–188
Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, Del Valle J, Pallas M (2014) Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis 42(4):1209–1220. doi:10.3233/JAD-140444
Pallas M, Porquet D, Vicente A, Sanfeliu C (2013) Resveratrol: new avenues for a natural compound in neuroprotection. Curr Pharm Des 19(38):6726–6731
Cox EP, O’Dwyer N, Cook R, Vetter M, Cheng HL, Rooney K, O’Connor H (2016) Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: a systematic review. J Sci Med Sport 19(8):616–628. doi:10.1016/j.jsams.2015.09.003
Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM (2011) Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav 104(5):934–941. doi:10.1016/j.physbeh.2011.06.005
Witte AV, Fobker M, Gellner R, Knecht S, Floel A (2009) Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A 106(4):1255–1260. doi:10.1073/pnas.0808587106
Witte AV, Kerti L, Margulies DS, Floel A (2014) Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci 34(23):7862–7870. doi:10.1523/JNEUROSCI.0385-14.2014
Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35(3):146–154. doi:10.1016/j.tips.2013.12.004
Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R (2014) SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13(5):787–796. doi:10.1111/acel.12220
Acknowledgments
This study was supported by Grants CSD2010-00045 and SAF2012-39852 from the Spanish Ministry of Economy and Competitiveness (MINECO) and the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Corpas, R., Revilla, S., Ursulet, S. et al. SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Mol Neurobiol 54, 5604–5619 (2017). https://doi.org/10.1007/s12035-016-0087-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0087-9