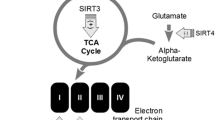
Abstract
Ageing is a stochastic process associated with a progressive decline in physiological functions which predispose to the pathogenesis of several neurodegenerative diseases. The intrinsic complexity of ageing remains a significant challenge to understand the cause of this natural phenomenon. At the molecular level, ageing is thought to be characterized by the accumulation of chronic oxidative damage to lipids, proteins and nucleic acids caused by free radicals. Increased oxidative stress and misfolded protein formations, combined with impaired compensatory mechanisms, may promote neurodegenerative disorders with age. Nutritional modulation through calorie restriction has been shown to be effective as an anti-ageing factor, promoting longevity and protecting against neurodegenerative pathology in yeast, nematodes and murine models. Calorie restriction increases the intracellular levels of the essential pyridine nucleotide, nicotinamide adenine dinucleotide (NAD+), a co-substrate for the sirtuin 1 (Sirt1, silent mating-type information regulator 2 homolog 1) activity and a cofactor for oxidative phosphorylation and ATP synthesis. Promotion of intracellular NAD+ anabolism is speculated to induce neuroprotective effects against amyloid-β-peptide (Aβ) toxicity in some models for Alzheimer’s disease (AD). The NAD+-dependent histone deacetylase, Sirt1, has been implicated in the ageing process. Sirt1 serves as a deacetylase for numerous proteins involved in several cellular pathways, including stress response and apoptosis, and plays a protective role in neurodegenerative disorders, such as AD.






Similar content being viewed by others
References
Lutz W, Sanderson W, Scherbov S (2008) The coming acceleration of global population ageing. Nature 451:716–719
Joseph JA, Shukitt-Hale B, Casadesus G (2005) Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr 81(1 Suppl):313S–316S
Rattan S (2006) Theories of biological aging: genes, proteins, and free radicals. Free Radic Res 40:1230–1238
Harman D (2006) Free radical theory of aging: an update. Ann NY Acad Sci 1067:10–21
Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280:26185–92
Massudi H, Grant R, Guillemin GJ, Braidy N (2012) NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Rep 17:28–46
Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ (2012) Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7:e42357
Braidy N, Guillemin G, Mansour H, Chan-Ling T, Poljak A, Grant R (2011) Age related changes in NAD+ metabolism, oxidative stress and Sirt1 activity in Wistar rats. PLoS One 6:e19194
Wong E, Cuervo AM (2010) Integration of clearance mechanism: the proteosome and autophagy. Cold Spring Harb Perspect Biol 2:a006734
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Bergamini E, Cavallini G, Donati A, Gori Z (2007) The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction. Ann N Y Acad Sci 1114:69–78
Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6:298–305
Hekimi S (2006) How genetic analysis tests theories of animal aging. Nat Genet 38:985–991
Jayasena T, Poljak A, Smythe G, Braidy N, Munch G, Sachdev P (2013) The role of polyphenols in the modulation of sirtuins and pathways involved in Alzheimer’s disease. Ageing Res Rev 12:867–883
Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes and Dev 20:2913–2921
Tanner KG, Landry J, Sternglanz, Denu JM (2000) Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A 97:14178–82
Fu XH, Meng FL, Hu Y, Zhou JQ (2008) Candida albicans, a distinctive fungal model for cellular aging study. Aging Cell 7:746–57
Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Chen J (2011) Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol 95:373–395
Herskovits AZ, Guarente L (2013) Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23:746–758
Cooper HM, Spelbrink JN (2008) The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J 411:279–85
Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G (2003) Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol 38:1065–70
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman VE, Schwer B (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27:8807–8814
Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C (2008) Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol 382:790–801
Hisahara S, Chiba S, Matsumoto H, Horio Y (2005) Transcriptional regulation of neuronal genes and its effect on neural functions: NAD-dependent histone deacetylase SIRT1 (Sir2alpha). J Pharmacol Sci 98:200–204
Blander G, Guarente L (2004) The Sir2 family of protein deacetylases. Annu Rev Biochem 73:417–435
Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305:1010–1013
Pallàs M, Pizarro JG, Gutierrez-Cuesta J, Crespo-Biel N, Alvira D, Tajes M, Yeste-Velasco M, Folch J, Canudas AM, Sureda FX, Ferrer I, Camins A (2008) Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience 154:1388–1397
Braidy N, Jayasena T, Poljak A, Sachdev P (2012) Sirtuins in cognitive ageing and Alzheimer’s disease. Curr Opin Psychiatry 25:226–30
Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37:349–50
Kim D, Nguyen MD, Dobbin M, Fischer A, Sananbenesi F, Rodgers J, Delalle I, Baur J, Sui G, Armour S, Puigserver P, Sinclair D, Tsai L (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO Journal 26:3169–3179
Chen D, Steele AD, Hutter G, Bruno J, Govindarajan A, Easlon E, Lin SJ, Aguzzi A, Lindquist S, Guarente L (2008) The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration. Exp Gerontol 43:1086–93
Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA (2008) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3:e2264
Yang Q, Guan KL (2007) Expanding mTOR signaling. Cell Res 17:666–681
Loeb LL, Wallace DC, Martin GM (2005) The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci U S A 102:18769–18770
Rattan SI (2006) Theories of biological aging: genes, proteins, and free radicals. Free Radic Res 40:1230–1238
Keating DJ (2008) Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative disease. J Neurochem 104:298–305
Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC (2000) The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog Neurobiol 62:633–48
Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3:431–443
Ueda Y, Doi T, Nagatomo K, Nakajima A (2007) In vivo activation of N-methyl-D-aspartate receptors generates free radicals and reduces antioxidant ability in the rat hippocampus: experimental protocol of in vivo ESR spectroscopy and microdialysis for redox status evaluation. Brain Res 1178:20–27
Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE (2008) Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11:476–487
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer’s-related amyloid β-oligomers. J Neurosci 24:10191–10200
Dinamarca MC, Ríos JA, Inestrosa NC (2012) Postsynaptic receptors for amyloid-β oligomers as mediators of neuronal damage in Alzheimer’s disease. Front Physiol 3:464
De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL (2007) Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem 282:11590–11601
Magnusson KR, Nelson SE, Young AB (2002) Age-related changes in the protein expression of subunits of the NMDA receptor. Mol Brain Res 99:40–45
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120:497–512
Hirano T, Yamaguchi R, Asami S, Iwamoto N, Kasai H (1996) 8-Hydroxyguanine levels in nuclear DNA and its repair activity in rat organs associated with age. J Gerontol A Biol Sci Med Sci 51:B303–B307
Shen S, Cooley DM, Glickman LT, Glickman N, Waters DJ (2001) Reduction in DNA damage in brain and peripheral blood lymphocytes of elderly dogs after treatment with dehydroepiandrosterone (DHEA). Mutat Res 480–481:153–162
Krishna TH, Mahipal S, Sudhakar A, Sugimoto H, Kalluri R, Rao KS (2005) Reduced DNA gap repair in aging rat neuronal extracts and its restoration by DNA polymerase beta and DNA-ligase. J Neurochem 92:818–823
Giovannelli L, Decorosi F, Dolara P, Pulvirenti L (2003) Vulnerability to DNA damage in the aging rat substantia nigra: a study with the comet assay. Brain Res 969:244–247
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883–891
Rutten BP, Schmitz C, Gerlach OH, Oyen HM, de Mesquita EB, Steinbusch HW, Korr H (2007) The aging brain: accumulation of DNA damage or neuron loss? Neurobiol Aging 28:91–98
Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A (2007) Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73:550–560
Bureau G, Longpré F, Martinoli MG (2008) Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res 86:403–10
Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, SY E, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339:1216–9
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306:990–995
Lum JJ, DeBerardinis RJ, Thompson CB (2005) Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol 6:439–448
Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243:240–246
Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK (2010) Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One 5:e12845
Yang H, Yang T, Baur JA, Perez, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130:1095–1107
Gouras GK (2013) mTOR: at the crossroads of aging, chaperones, and Alzheimer’s disease. J Neurochem 124(6):747–748. doi:10.1111/jnc.12098
Cai Z, Yan LJ (2013) Rapamycin, autophagy, and Alzheimer’s disease. J Biochem Pharmacol Res 1:84–90
Urbanska M, Gozdz A, Swiech LJ, Jaworski J (2012) Mammalian target of rapamycin complex 1 (MTORC1) and 2 (MTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem 287:30240–56
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007) mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450:736–740
Terman A (1995) The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41:319–26
Kurz T, Terman A, Gustafsson B, Brunk UT (2008) Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta 1780:1291–303
Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM (2012) Development and characterization of a new Parkinson’s disease model resulting from impaired autophagy. J Neurosci 14:16503–9
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z (2007) Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A 104:14489–14494
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105:3374–3379
Raghavan A, Shah ZA (2012) Sirtuins in neurodegenerative diseases: a biological-chemical perspective. Neurodegenerative Dis 9:1–10
McBurney MW, Clark-Knowles KV, Caron AZ, Gray DA (2013) SIRT1 is a highly networked protein that mediates the adaptation to chronic physiological stress. Genes Cancer 4:125–34
King MA, Hands S, Hafiz F, Mizushima N, Tolkovsky AM, Wyttenbach A (2008) Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol 73:1052–1063
Drachman DA (2006) Aging of the brain, entropy, and Alzheimer disease. Neurology 67:1340–1352
Stark AK, Toft MH, Pakkenberg H, Fabricius K, Eriksen N, Pelvig DP, Moller M, Pakkenberg B (2007) The effect of age and gender on the volume and size distribution of neocortical neurons. Neuroscience 150:121–130
Sherwood CC, Gordon AD, Allen JS, Phillips KA, Hof PR, Hopkins WD (2011) Aging of the cerebral cortex differs between humans and chimpanzees. Proc Natl Acad Sci U S A 108:13029–12034
Rapp PR, Deroche PS, Mao Y, Burwell RD (2002) Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex 12:1171–1179
Burke SN, Ryan L, Barnes CA (2012) Characterizing cognitive aging of recognition memory and related processes in animal models and in humans. Front Aging Neurosci 4:15
Virgili M, Monti B, Polazzi E, Angiolini G, Contestabile A (2001) Topography of neurochemical alterations in the CNS of aged rats. Int J Dev Neurosci 19:109–116
Monti B, Virgili M, Contestabile A (2004) Alterations of markers related to synaptic function in aging rat brain, in normal conditions or under conditions of long-term dietary manipulation. Neurochem Int 44:579–84
Eckles-Smith K, Clayton D, Bickford P, Browning MD (2000) Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res 78:154–162
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93:884S–890S
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280:16456–16460
Yamamoto T, Shimano H, Nakagawa Y, Ide T, Yahagi N, Matsuzaka T, Nakakuki M, Takahashi A, Suzuki H, Sone H, Toyoshima H, Sato R, Yamada N (2004) SREBP-1 interacts with hepatocyte nuclear factor-4 alpha and interferes with PGC-1 recruitment to suppress hepatic gluconeogenic genes. J Bol Chem 279:12027–35
Zolezzi JM, Silva-Alvarez C, Ordenes D, Godoy JA, Carvajal FJ, Santos MJ, Inestrosa NC (2013) Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One 8:e64019
Itoh K, Nakamura K, Iijima M, Sesaki H (2012) Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23:64–71
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460:587–591
Sack MN, Finkel T (2012) Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol 4:1–10. doi:10.1101/cshperspect.a013102
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–90
Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6:1054–1061
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14:45–53
Haass C, Selkoe D (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8:101–112
Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE (2005) Caloric restriction attenuates Aβ deposition in Alzheimer transgenic models. Neurobiol Aging 26:995–1000
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754
Alvarez VA, Sabatini BL (2007) Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30:79–97
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12:2685–705
Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–707
Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Curr Opin Genet Dev 12:14–21
Codocedo JF, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC (2012) SIRT1 regulates dendritic development in hippocampal neurons. PLoS One 7:e47073. doi:10.1371/journal.pone.0047073
Negishi M, Katoh H (2002) Rho family GTPases as key regulators for neuronal network formation. J Biochem 132:157–66
Duan W, Mattson MP (1999) Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res 57:195–206
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J Biol Chem 280:37377–37382
Wu A, Ying Z, Gomez-Pinilla F (2006) Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci 23:2573–2580
Acknowledgements
This work was supported by grants PFB 12/2007 from the Basal Centre for Excellence in Science and Technology, FONDECYT 1120156 and MIFAB Institute and Fundación Ciencia y Vida to NCI and FONDECYT No. 11130033 to JMZ. NB is a recipient of the Alzheimer’s Australia and NHMRC Early Career Postdoctoral Research Fellowship at the University of New South Wales, Sydney, Australia.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Godoy, J.A., Zolezzi, J.M., Braidy, N. et al. Role of Sirt1 During the Ageing Process: Relevance to Protection of Synapses in the Brain. Mol Neurobiol 50, 744–756 (2014). https://doi.org/10.1007/s12035-014-8645-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8645-5